Page 969 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 969
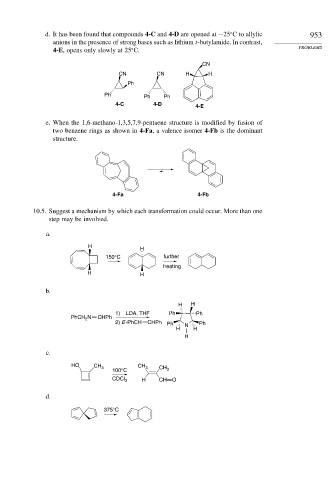
d. It has been found that compounds 4-C and 4-D are opened at −25 C to allylic 953
anions in the presence of strong bases such as lithium t-butylamide. In contrast,
PROBLEMS
4-E, opens only slowly at 25 C.
CN
CN CN H H
Ph
Ph Ph Ph
4-C 4-D 4-E
e. When the 1,6-methano-1,3,5,7,9-pentaene structure is modified by fusion of
two benzene rings as shown in 4-Fa, a valence isomer 4-Fb is the dominant
structure.
4-Fa 4-Fb
10.5. Suggest a mechanism by which each transformation could occur. More than one
step may be involved.
a.
H
H
150°C further
heating
H H
b.
H H
1) LDA, THF Ph Ph
PhCH N CHPh
2
2) E -PhCH CHPh Ph Ph
H N H
H
c.
HO CH 3 CH 3 CH
100°C 3
CDCl 3 H CH O
d.
375°C