Page 972 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 972
956 10.9. Bromocyclooctatetraene rearranges to E- -bromostyrene. The rate of the
rearrangement is solvent dependent, with the first-order rate constant increasing
CHAPTER 10 from about 10 −7 −1 in cyclohexane to about 10 −3 −1 in acetonitrile at 80 C.
s
s
Concerted Pericyclic In the presence of lithium iodide, the product is E- -iodostyrene, although
Reactions
E- -bromostyrene is unaffected by lithium iodide under the reaction conditions.
Suggest a mechanism for the rearrangement.
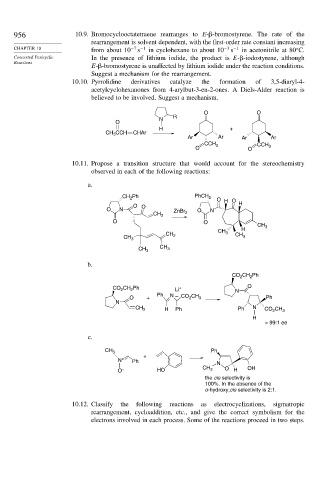
10.10. Pyrrolidine derivatives catalyze the formation of 3,5-diaryl-4-
acetylcyclohexanones from 4-arylbut-3-en-2-ones. A Diels-Alder reaction is
believed to be involved. Suggest a mechanism.
O O
R
N
O
H +
CH 3 CCH CHAr
Ar Ar Ar Ar
CCH 3 CCH 3
O O
10.11. Propose a transition structure that would account for the stereochemistry
observed in each of the following reactions:
a.
CH Ph PhCH 2 O H O
2
O O H
O N ZnBr O N
CH 2 2
O O
CH 3
CH H
CH 3 CH
CH 3 2 3
CH 3 CH 3
b.
CH Ph
CO 2 2
CO CH Ph Li + N O
2
2
Ph N –
O + CO CH 3 Ph
2
N
CH 2 H Ph Ph N CO CH 3
2
H
> 99:1 ee
c.
CH 3 Ph
+
N + Ph
N
O – HO CH 3 O H OH
the cis selectivity is
100%. In the absence of the
o-hydroxy,cis selectivity is 2:1.
10.12. Classify the following reactions as electrocyclizations, sigmatropic
rearrangement, cycloaddition, etc., and give the correct symbolism for the
electrons involved in each process. Some of the reactions proceed in two steps.