Page 974 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 974
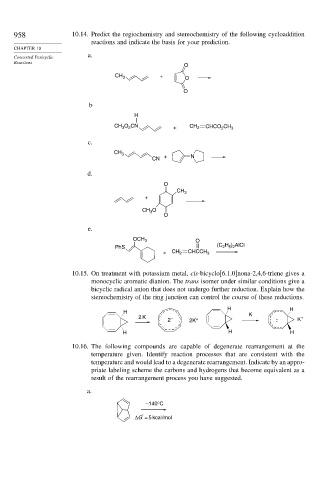
958 10.14. Predict the regiochemistry and stereochemistry of the following cycloaddition
reactions and indicate the basis for your prediction.
CHAPTER 10
a.
Concerted Pericyclic
Reactions
O
CH 3 + O
O
b
H
CH O CN + CH 2 CHCO 2 CH 3
2
3
c.
CH 3
CN + N
d.
O
CH 3
+
CH O
3
O
e.
OCH 3 O
H ) AlCl
PhS (C 2 5 2
+ CH 2 CHCCH 3
10.15. On treatment with potassium metal, cis-bicyclo[6.1.0]nona-2,4,6-triene gives a
monocyclic aromatic dianion. The trans isomer under similar conditions give a
bicyclic radical anion that does not undergo further reduction. Explain how the
stereochemistry of the ring junction can control the course of these reductions.
H H
H K
2 K – +
2 2K + - . K
H H H
10.16. The following compounds are capable of degenerate rearrangement at the
temperature given. Identify reaction processes that are consistent with the
temperature and would lead to a degenerate rearrangement. Indicate by an appro-
priate labeling scheme the carbons and hydrogens that become equivalent as a
result of the rearrangement process you have suggested.
a.
–140°C
*
ΔG = 5 kcal/mol