Page 975 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 975
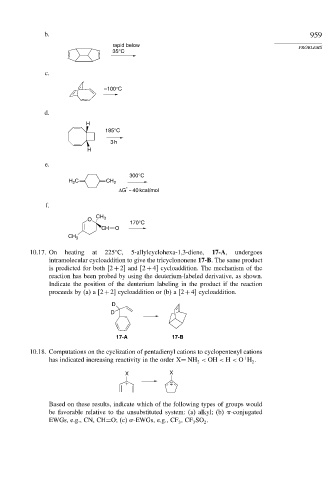
b. 959
rapid below PROBLEMS
35°C
c.
–100°C
d.
H
185°C
3 h
H
e.
300°C
H C CH 2
2
*
ΔG ~ 40 kcal/mol
f.
CH
O 3
170°C
CH O
CH 3
10.17. On heating at 225 C, 5-allylcyclohexa-1,3-diene, 17-A, undergoes
intramolecular cycloaddition to give the tricyclononene 17-B. The same product
is predicted for both [2 + 2] and [2 + 4] cycloaddition. The mechanism of the
reaction has been probed by using the deuterium-labeled derivative, as shown.
Indicate the position of the deuterium labeling in the product if the reaction
proceeds by (a) a [2+2] cycloaddition or (b) a [2+4] cycloaddition.
D
D
17-A 17-B
10.18. Computations on the cyclization of pentadienyl cations to cyclopentenyl cations
+
has indicated increasing reactivity in the order X= NH < OH < H < O H .
2
2
X X
+ +
Based on these results, indicate which of the following types of groups would
be favorable relative to the unsubstituted system: (a) alkyl; (b) -conjugated
EWGs, e.g., CN, CH=O; (c) -EWGs, e.g., CF ,CF SO .
2
3
3