Page 978 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 978
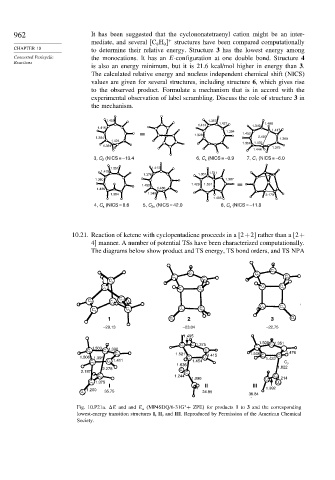
962 It has been suggested that the cyclononatetraenyl cation might be an inter-
+
mediate, and several [C H structures have been compared computationally
9 9
CHAPTER 10 to determine their relative energy. Structure 3 has the lowest energy among
Concerted Pericyclic the monocations. It has an E-configuration at one double bond. Structure 4
Reactions
is also an energy minimum, but it is 21.6 kcal/mol higher in energy than 3.
The calculated relative energy and nucleus independent chemical shift (NICS)
values are given for several structures, including structure 6, which gives rise
to the observed product. Formulate a mechanism that is in accord with the
experimental observation of label scrambling. Discuss the role of structure 3 in
the mechanism.
1.409 1.357
1.474 1.427 1.349 1.488
1.416
1.394 1.417
1.328 1.452
1.394 2.197 1.399
1.424
1.354 1.435
1.381 1.375
1.444
3, C 2 (NICS = –13.4 6, C s (NICS = –0.9 7, C 1 (NICS = –6.0
1.351 1.413
1.479 1.511
1.376 1.361
1.360 1.387
1.455 1.428 1.557
1.436 1.480
1.384 1.349 2.179
1.495
4, C s (NICS = 8.6 5, C 2v (NICS = 42.0 8, C s (NICS = –11.8
10.21. Reaction of ketene with cyclopentadiene proceeds in a [2+2] rather than a [2+
4] manner. A number of potential TSs have been characterized computationally.
The diagrams below show product and TS energy, TS bond orders, and TS NPA
C
C C 4
C 4 5 C
C 5 C 3 3
5
C C C 1 C
1 C 2 2
1
C C 7 O
2 C 8
4 C C
C C 6 C 6 C 7 6 7
3
1 O 8 2 3 O 8
–29.13 –23.04 –22.75
1.495
C C 1.375 1.508 C 4 1.351
1.503 1.396 5 4 C 5
C C C
5 4 C 1.521 3 1.415 1.506 1.476
1.506 1.395 3 C 1 C 2 C 1 1.422 C 3
C C 1.411 1.454 C
1 2 1.636 2
2.278 1.822
2.187 C 6 C
C 1.244 7 C
6 1.390 7 1.214
C 1.375 C 6 O
7 8
I O II III
1.200 8 1.392
O 35.75 24.95
8 36.84
∗
Fig. 10.P21a. E and and E a (MP4SDQ/6-31G + ZPE) for products 1 to 3 and the corresponding
lowest-energy transition structures I, II, and III. Reproduced by Permission of the American Chemical
Society.