Page 988 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 988
972
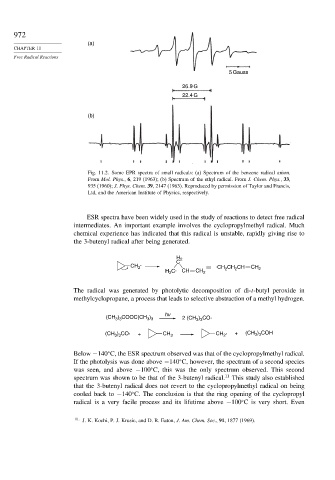
(a)
CHAPTER 11
Free Radical Reactions
5 Gauss
26.9 G
22.4 G
(b)
Fig. 11.2. Some EPR spectra of small radicals: (a) Spectrum of the benzene radical anion.
From Mol. Phys., 6, 219 (1963); (b) Spectrum of the ethyl radical. From J. Chem. Phys., 33,
935 (1960); J. Phys. Chem. 39, 2147 (1963). Reproduced by permission of Taylor and Francis,
Ltd, and the American Institute of Physics, respectively.
ESR spectra have been widely used in the study of reactions to detect free radical
intermediates. An important example involves the cyclopropylmethyl radical. Much
chemical experience has indicated that this radical is unstable, rapidly giving rise to
the 3-butenyl radical after being generated.
H 2
C
CH 2 . . CH 2 CH CH CH
H C . CH CH 2 2 2
2
The radical was generated by photolytic decomposition of di-t-butyl peroxide in
methylcyclopropane, a process that leads to selective abstraction of a methyl hydrogen.
hv
(CH ) COOC(CH ) 2 (CH ) CO·
3 3
3 3
3 3
) COH
(CH ) CO· + CH 3 CH · + (CH 3 3
2
3 3
Below −140 C, the ESR spectrum observed was that of the cyclopropylmethyl radical.
If the photolysis was done above −140 C, however, the spectrum of a second species
was seen, and above −100 C, this was the only spectrum observed. This second
spectrum was shown to be that of the 3-butenyl radical. 11 This study also established
that the 3-butenyl radical does not revert to the cyclopropylmethyl radical on being
cooled back to −140 C. The conclusion is that the ring opening of the cyclopropyl
radical is a very facile process and its lifetime above −100 C is very short. Even
11
J. K. Kochi, P. J. Krusic, and D. R. Eaton, J. Am. Chem. Soc., 91, 1877 (1969).