Page 989 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 989
though the equilibrium favors the 3-butenyl radical, the reversible ring closure can be 973
detected by an isotopic-labeling experiment that reveals the occurrence of deuterium
exchange. SECTION 11.1
Generation and
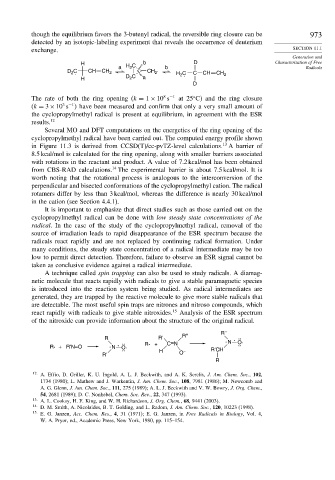
H H C b D Characterization of Free
a 2 b Radicals
D 2 C CH CH CH ·
· 2 D 2 C 2 H C C CH CH 2
2 ·
H a
D
8 −1
The rate of both the ring opening (k = 1 × 10 s at 25 C) and the ring closure
3 −1
(k = 3 × 10 s
have been measured and confirm that only a very small amount of
the cyclopropylmethyl radical is present at equilibrium, in agreement with the ESR
results. 12
Several MO and DFT computations on the energetics of the ring opening of the
cyclopropylmethyl radical have been carried out. The computed energy profile shown
13
in Figure 11.3 is derived from CCSD(T)/cc-pvTZ-level calculations. A barrier of
8.5 kcal/mol is calculated for the ring opening, along with smaller barriers associated
with rotations in the reactant and product. A value of 7.2 kcal/mol has been obtained
14
from CBS-RAD calculations. The experimental barrier is about 7.5 kcal/mol. It is
worth noting that the rotational process is analogous to the interconversion of the
perpendicular and bisected conformations of the cyclopropylmethyl cation. The radical
rotamers differ by less than 3 kcal/mol, whereas the difference is nearly 30 kcal/mol
in the cation (see Section 4.4.1).
It is important to emphasize that direct studies such as those carried out on the
cyclopropylmethyl radical can be done with low steady state concentrations of the
radical. In the case of the study of the cyclopropylmethyl radical, removal of the
source of irradiation leads to rapid disappearance of the ESR spectrum because the
radicals react rapidly and are not replaced by continuing radical formation. Under
many conditions, the steady state concentration of a radical intermediate may be too
low to permit direct detection. Therefore, failure to observe an ESR signal cannot be
taken as conclusive evidence against a radical intermediate.
A technique called spin trapping can also be used to study radicals. A diamag-
netic molecule that reacts rapidly with radicals to give a stable paramagnetic species
is introduced into the reaction system being studied. As radical intermediates are
generated, they are trapped by the reactive molecule to give more stable radicals that
are detectable. The most useful spin traps are nitrones and nitroso compounds, which
react rapidly with radicals to give stable nitroxides. 15 Analysis of the ESR spectrum
of the nitroxide can provide information about the structure of the original radical.
R''
R''
R R' = · N · ··
O·
R· + R'N=O · N · ·· R· + C N ··
O·
·· H – R'CH
R' O
R
12 A. Effio, D. Griller, K. U. Ingold, A. L. J. Beckwith, and A. K. Serelis, J. Am. Chem. Soc., 102,
1734 (1980); L. Mathew and J. Warkentin, J. Am. Chem. Soc., 108, 7981 (1986); M. Newcomb and
A. G. Glenn, J. Am. Chem. Soc., 111, 275 (1989); A. L. J. Beckwith and V. W. Bowry, J. Org. Chem.,
54, 2681 (1989); D. C. Nonhebel, Chem. Soc. Rev., 22, 347 (1993).
13 A. L. Cooksy, H. F. King, and W. H. Richardson, J. Org. Chem., 68, 9441 (2003).
14 D. M. Smith, A. Nicolaides, B. T. Golding, and L. Radom, J. Am. Chem. Soc., 120, 10223 (1998).
15
E. G. Janzen, Acc. Chem. Res., 4, 31 (1971); E. G. Janzen, in Free Radicals in Biology, Vol. 4,
W. A. Pryor, ed., Academic Press, New York, 1980, pp. 115–154.