Page 991 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 991
nuclear spin states dictated by the Boltzmann distribution is disturbed by the presence 975
of an unpaired electron. The magnetic moment associated with an electron causes
a redistribution of the nuclear spin states. Molecules can become overpopulated in SECTION 11.1
either the lower or upper spin state. If the lower state is overpopulated an enhanced Generation and
Characterization of Free
absorption signal is observed. If the upper state is overpopulated, an emission signal Radicals
is observed. The CIDNP method is not as general as EPR spectroscopy because not
all free radical reactions can be expected to exhibit the phenomenon. 17
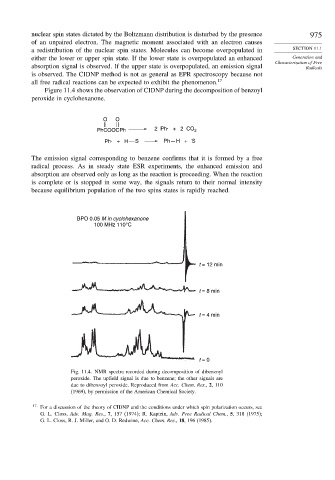
Figure 11.4 shows the observation of CIDNP during the decomposition of benzoyl
peroxide in cyclohexanone.
O O
PhCOOCPh 2 Ph· + 2 CO 2
·
Ph· + H S Ph H + S
The emission signal corresponding to benzene confirms that it is formed by a free
radical process. As in steady state ESR experiments, the enhanced emission and
absorption are observed only as long as the reaction is proceeding. When the reaction
is complete or is stopped in some way, the signals return to their normal intensity
because equilibrium population of the two spins states is rapidly reached.
BPO 0.05 M in cyclohexanone
100 MHz 110°C
t = 12 min
t = 8 min
t = 4 min
t = 0
Fig. 11.4. NMR spectra recorded during decomposition of dibenzoyl
peroxide. The upfield signal is due to benzene; the other signals are
due to dibenzoyl peroxide. Reproduced from Acc. Chem. Res., 2, 110
(1969), by permission of the American Chemical Society.
17
For a discussion of the theory of CIDNP and the conditions under which spin polarization occurs, see
G. L. Closs, Adv. Mag. Res., 7, 157 (1974); R. Kaptein, Adv. Free Radical Chem., 5, 318 (1975);
G. L. Closs, R. J. Miller, and O. D. Redwine, Acc. Chem. Res., 18, 196 (1985).