Page 996 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 996
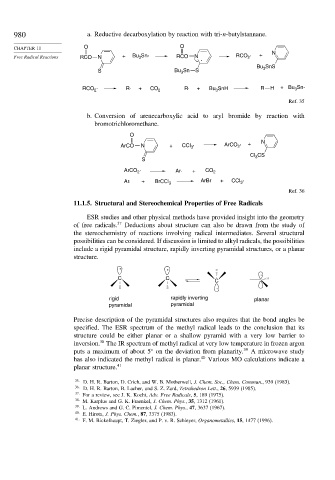
980 a. Reductive decarboxylation by reaction with tri-n-butylstannane.
CHAPTER 11 O O
Free Radical Reactions RCO N + Bu Sn· RCO N RCO · + N
2
3
.
Bu SnS
3
S Bu Sn S
3
RCO · R· + CO 2 R· + Bu SnH R H + Bu Sn·
3
3
2
Ref. 35
b. Conversion of arenecarboxylic acid to aryl bromide by reaction with
bromotrichloromethane.
O
ArCO N + CCl · ArCO 2 · + N
3
CS
Cl 3
S
ArCO · Ar· + CO 2
2
Ar· + BrCCl 3 ArBr + CCl 3 ·
Ref. 36
11.1.5. Structural and Stereochemical Properties of Free Radicals
ESR studies and other physical methods have provided insight into the geometry
of free radicals. 37 Deductions about structure can also be drawn from the study of
the stereochemistry of reactions involving radical intermediates. Several structural
possibilities can be considered. If discussion is limited to alkyl radicals, the possibilities
include a rigid pyramidal structure, rapidly inverting pyramidal structures, or a planar
structure.
· ·
.
C C
C
rigid rapidly inverting . planar
pyramidal pyramidal
Precise description of the pyramidal structures also requires that the bond angles be
specified. The ESR spectrum of the methyl radical leads to the conclusion that its
structure could be either planar or a shallow pyramid with a very low barrier to
38
inversion. The IR spectrum of methyl radical at very low temperature in frozen argon
puts a maximum of about 5 on the deviation from planarity. 39 A microwave study
has also indicated the methyl radical is planar. 40 Various MO calculations indicate a
planar structure. 41
35 D. H. R. Barton, D. Crich, and W. B. Motherwell, J. Chem. Soc., Chem. Commun., 939 (1983).
36
D. H. R. Barton, B. Lacher, and S. Z. Zard, Tetrahedron Lett., 26, 5939 (1985).
37 For a review, see J. K. Kochi, Adv. Free Radicals, 5, 189 (1975).
38
M. Karplus and G. K. Fraenkel, J. Chem. Phys., 35, 1312 (1961).
39
L. Andrews and G. C. Pimentel, J. Chem. Phys., 47, 3637 (1967).
40 E. Hirota, J. Phys. Chem., 87, 3375 (1983).
41
F. M. Bickelhaupt, T. Ziegler, and P. v. R. Schleyer, Organometallics, 15, 1477 (1996).