Page 998 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 998
982 The tendency for pyramidal geometry is reinforced by an interaction between the p
orbital on carbon and the antibonding orbitals associated with the C−ForC−O
∗
CHAPTER 11
bonds. The interaction increases electron density on the more electronegative fluorine
Free Radical Reactions or oxygen atom. This stabilizing p- interaction is increased by pyramidal geometry.
∗
.
. F
F C X
F
X X
pyramidalization reduces electron-electron
repulsion and enhances p −σ* interaction
. . .
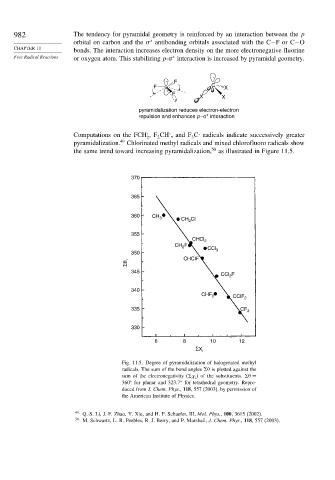
Computations on the FCH ,F CH , and F C radicals indicate successively greater
2 2 3
49
pyramidalization. Chlorinated methyl radicals and mixed chlorofluoro radicals show
the same trend toward increasing pyramidalization, 50 as illustrated in Figure 11.5.
370
365
360
CH 2 Cl
CH 3
355
CHCl 2
CH 2 F
CCl 3
350
CHClF
Σθ i
345
CCl 2 F
340
CHF 2
CClF 2
335 CF 3
330
6 8 10 12
ΣX i
Fig. 11.5. Degree of pyramidalization of halogenated methyl
radicals. The sum of the bond angles
is plotted against the
sum of the electronegativity (
i
of the substituents.
=
360 for planar and 323 7 for tetrahedral geometry. Repro-
duced from J. Chem. Phys., 118, 557 (2003), by permission of
the American Institute of Physics.
49 Q.-S. Li, J.-F. Zhao, Y. Xie, and H. F. Schaefer, III, Mol. Phys., 100, 3615 (2002).
50
M. Schwartz, L. R. Peebles, R. J. Berry, and P. Marshall, J. Chem. Phys., 118, 557 (2003).