Page 1007 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1007
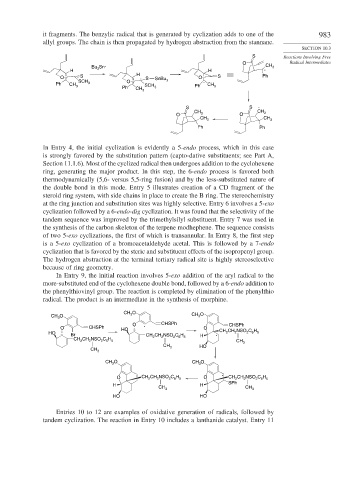
it fragments. The benzylic radical that is generated by cyclization adds to one of the 983
allyl groups. The chain is then propagated by hydrogen abstraction from the stannane.
SECTION 10.3
S Reactions Involving Free
O Radical Intermediates
Bu Sn . CH 3
H 3 H .
S H S Ph
O . S SnBu O
O 3 .
SCH 3
Ph CH 3 Ph CH
Ph CH 3 SCH 3 3
S S .
O CH 3 O CH 2
CH CH 3
3
Ph Ph
In Entry 4, the initial cyclization is evidently a 5-endo process, which in this case
is strongly favored by the substitution pattern (capto-dative substituents; see Part A,
Section 11.1.6). Most of the cyclized radical then undergoes addition to the cyclohexene
ring, generating the major product. In this step, the 6-endo process is favored both
thermodynamically (5,6- versus 5,5-ring fusion) and by the less-substituted nature of
the double bond in this mode. Entry 5 illustrates creation of a CD fragment of the
steroid ring system, with side chains in place to create the B ring. The stereochemistry
at the ring junction and substitution sites was highly selective. Entry 6 involves a 5-exo
cyclization followed by a 6-endo-dig cyclization. It was found that the selectivity of the
tandem sequence was improved by the trimethylsilyl substituent. Entry 7 was used in
the synthesis of the carbon skeleton of the terpene modhephene. The sequence consists
of two 5-exo cyclizations, the first of which is transannular. In Entry 8, the first step
isa5-exo cyclization of a bromoacetaldehyde acetal. This is followed by a 7-endo
cyclization that is favored by the steric and substituent effects of the isopropenyl group.
The hydrogen abstraction at the terminal tertiary radical site is highly stereoselective
because of ring geometry.
In Entry 9, the initial reaction involves 5-exo addition of the aryl radical to the
more-substituted end of the cyclohexene double bond, followed by a 6-endo addition to
the phenylthiovinyl group. The reaction is completed by elimination of the phenylthio
radical. The product is an intermediate in the synthesis of morphine.
CH O
CH O 3 CH 3 O
3
O . CHSPh CHSPh
O CHSPh HO O
2
6
HO Br CH NSO C H H . CH 2 CH NSO C H 5
2
CH CH NSO C H 5 CH 2 2 2 6 5 CH
2
2
6
2
CH HO 3
CH 3
3
CH O CH O
3
3
.
O CH CH NSO C H 5 O CH 2 CH NSO C H 5
2
6
2
6
2
2
2
SPh
H H
CH 3 CH 3
HO HO
Entries 10 to 12 are examples of oxidative generation of radicals, followed by
tandem cyclization. The reaction in Entry 10 includes a lanthanide catalyst. Entry 11