Page 1008 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1008
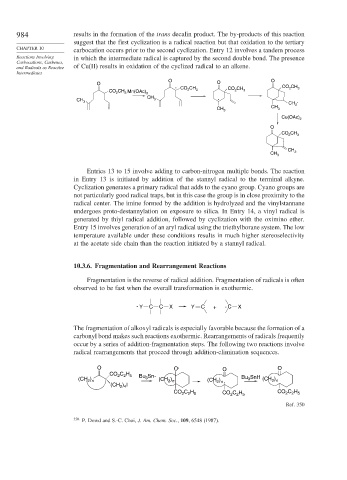
984 results in the formation of the trans decalin product. The by-products of this reaction
suggest that the first cyclization is a radical reaction but that oxidation to the tertiary
CHAPTER 10 carbocation occurs prior to the second cyclization. Entry 12 involves a tandem process
Reactions Involving in which the intermediate radical is captured by the second double bond. The presence
Carbocations, Carbenes,
and Radicals as Reactive of Cu(II) results in oxidation of the cyclized radical to an alkene.
Intermediates
O O
O O
. CH CO 2 CH
CO 2 CO CH 3
CH Mn(OAc) 3 2 3
CO 2 3 3
CH
CH 3 3 . CH .
CH 2
CH 3 3
Cu(OAc) 2
O
CH
CO 2 3
CH
CH 2
3
Entries 13 to 15 involve adding to carbon-nitrogen multiple bonds. The reaction
in Entry 13 is initiated by addition of the stannyl radical to the terminal alkyne.
Cyclization generates a primary radical that adds to the cyano group. Cyano groups are
not particularly good radical traps, but in this case the group is in close proximity to the
radical center. The imine formed by the addition is hydrolyzed and the vinylstannane
undergoes proto-destannylation on exposure to silica. In Entry 14, a vinyl radical is
generated by thiyl radical addition, followed by cyclization with the oximino ether.
Entry 15 involves generation of an aryl radical using the triethylborane system. The low
temperature available under these conditions results in much higher stereoselectivity
at the acetate side chain than the reaction initiated by a stannyl radical.
10.3.6. Fragmentation and Rearrangement Reactions
Fragmentation is the reverse of radical addition. Fragmentation of radicals is often
observed to be fast when the overall transformation is exothermic.
. Y C C X Y C + . C X
The fragmentation of alkoxyl radicals is especially favorable because the formation of a
carbonyl bond makes such reactions exothermic. Rearrangements of radicals frequently
occur by a series of addition-fragmentation steps. The following two reactions involve
radical rearrangements that proceed through addition-elimination sequences.
O O . O O
CO C H .
2 2 5
3
) Bu Sn (CH ) Bu SnH (CH )
3
2 n .
(CH 2 n 2 n (CH ) 2 n
(CH ) I
2 4
CO C H CO C H CO C H
2 2 5
2 2 5
2 2 5
Ref. 350
350
P. Dowd and S.-C. Choi, J. Am. Chem. Soc., 109, 6548 (1987).