Page 1010 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1010
986 alkyne and cyano groups, which is due to the additional strain introduced in the three-
2
membered ring by the sp carbon. Aryl groups are also relatively unreactive because
CHAPTER 10 of the loss of aromaticity in the cyclic intermediate.
Reactions Involving
Carbocations, Carbenes,
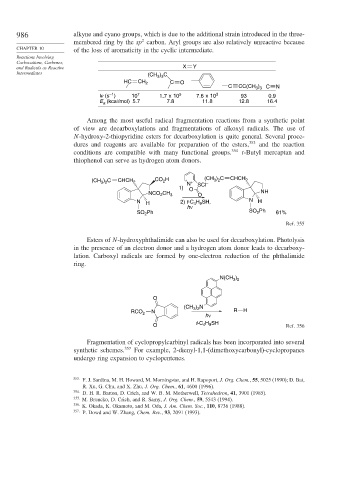
and Radicals as Reactive X Y
Intermediates (CH ) C
3 3
HC CH 2 C O
C CC(CH ) N
3 3 C
–1
kr (s ) 10 7 1.7 x 10 5 7.6 x 10 3 93 0.9
E (kcal/mol) 5.7 7.8 11.8 12.8 16.4
a
Among the most useful radical fragmentation reactions from a synthetic point
of view are decarboxylations and fragmentations of alkoxyl radicals. The use of
N-hydroxy-2-thiopyridine esters for decarboxylation is quite general. Several proce-
dures and reagents are available for preparation of the esters, 353 and the reaction
conditions are compatible with many functional groups. 354 t-Butyl mercaptan and
thiophenol can serve as hydrogen atom donors.
(CH ) C CHCH 2 CO H N + (CH ) C CHCH 2
3 2
2
3 2
–
1) O SCl
NCO CH 3 O NH
2
N H 2) t-C H SH, N H
4 9
hν
SO Ph SO Ph 61%
2
2
Ref. 355
Esters of N-hydroxyphthalimide can also be used for decarboxylation. Photolysis
in the presence of an electron donor and a hydrogen atom donor leads to decarboxy-
lation. Carboxyl radicals are formed by one-electron reduction of the phthalimide
ring.
N(CH )
3 2
O
(CH ) N
3 2
RCO 2 N hν R H
t-C 4 H 9 SH
O Ref. 356
Fragmentation of cyclopropylcarbinyl radicals has been incorporated into several
synthetic schemes. 357 For example, 2-dienyl-1,1-(dimethoxycarbonyl)-cyclopropanes
undergo ring expansion to cyclopentenes.
353
F. J. Sardina, M. H. Howard, M. Morningstar, and H. Rapoport, J. Org. Chem., 55, 5025 (1990); D. Bai,
R. Xu, G. Chu, and X. Zhu, J. Org. Chem., 61, 4600 (1996).
354
D. H. R. Barton, D. Crich, and W. B. M. Motherwell, Tetrahedron, 41, 3901 (1985).
355 M. Bruncko, D. Crich, and R. Samy, J. Org. Chem., 59, 5543 (1994).
356 K. Okada, K. Okamoto, and M. Oda, J. Am. Chem. Soc., 110, 8736 (1988).
357
P. Dowd and W. Zhang, Chem. Rev., 93, 2091 (1993).