Page 1013 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1013
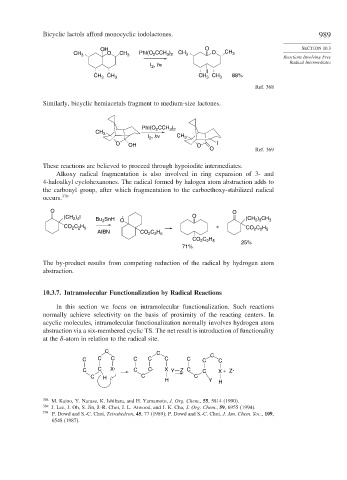
Bicyclic lactols afford monocyclic iodolactones. 989
OH O SECTION 10.3
CH 3 O CH 3 PhI(O CCH ) CH 3 O CH 3
2
3 2
Reactions Involving Free
I , hν Radical Intermediates
2
I
CH CH CH 88%
CH 3 3 3 3
Ref. 368
Similarly, bicyclic hemiacetals fragment to medium-size lactones.
CCH )
PhI(O 2 3 2
CH 3 , hν
I 2 CH 3
O OH O I
O Ref. 369
These reactions are believed to proceed through hypoiodite intermediates.
Alkoxy radical fragmentation is also involved in ring expansion of 3- and
4-haloalkyl cyclohexanones. The radical formed by halogen atom abstraction adds to
the carbonyl group, after which fragmentation to the carboethoxy-stabilized radical
occurs. 370
O O
(CH ) I Bu SnH O . O (CH ) CH 3
2 4
2 3
3
CO C H + CO C H
2 2 5
C H
AIBN CO 2 2 5 2 2 5
C H
CO 2 2 5
25%
71%
The by-product results from competing reduction of the radical by hydrogen atom
abstraction.
10.3.7. Intramolecular Functionalization by Radical Reactions
In this section we focus on intramolecular functionalization. Such reactions
normally achieve selectivity on the basis of proximity of the reacting centers. In
acyclic molecules, intramolecular functionalization normally involves hydrogen atom
abstraction via a six-membered cyclic TS. The net result is introduction of functionality
at the -atom in relation to the radical site.
C C
C C C C C C C C C C
C C X . C C . X Y Z C C X + Z .
C H C C
H Y H
368 M. Kaino, Y. Naruse, K. Ishihara, and H. Yamamoto, J. Org. Chem., 55, 5814 (1990).
369 J. Lee, J. Oh, S. Jin, J.-R. Choi, J. L. Atwood, and J. K. Cha, J. Org. Chem., 59, 6955 (1994).
370
P. Dowd and S.-C. Choi, Tetrahedron, 45, 77 (1989); P. Dowd and S.-C. Choi, J. Am. Chem. Soc., 109,
6548 (1987).