Page 1019 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1019
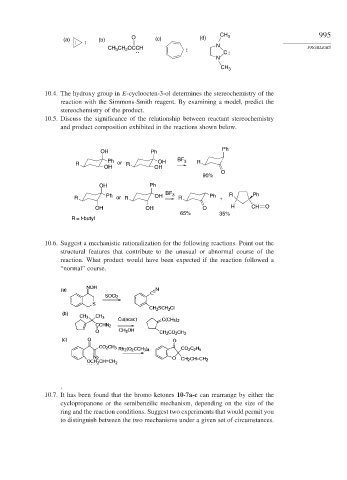
CH 3 995
(a) (b) O (c) (d)
:
CH CH OCCH : N PROBLEMS
2
3
C :
:
N
CH 3
10.4. The hydroxy group in E-cycloocten-3-ol determines the stereochemistry of the
reaction with the Simmons-Smith reagent. By examining a model, predict the
stereochemistry of the product.
10.5. Discuss the significance of the relationship between reactant stereochemistry
and product composition exhibited in the reactions shown below.
Ph
OH Ph
Ph OH BF 3
R or R R
OH OH
O
90%
OH Ph
Ph OH BF 3 Ph R Ph
R or R R +
OH OH O H CH O
65% 35%
R = t-butyl
10.6. Suggest a mechanistic rationalization for the following reactions. Point out the
structural features that contribute to the unusual or abnormal course of the
reaction. What product would have been expected if the reaction followed a
“normal” course.
.
10.7. It has been found that the bromo ketones 10-7a-c can rearrange by either the
cyclopropanone or the semibenzilic mechanism, depending on the size of the
ring and the reaction conditions. Suggest two experiments that would permit you
to distinguish between the two mechanisms under a given set of circumstances.