Page 1026 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1026
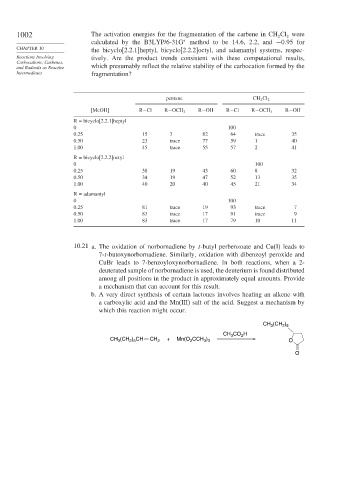
1002 The activation energies for the fragmentation of the carbene in CH Cl were
2
2
calculated by the B3LYP/6-31G method to be 14.6, 2.2, and −0 95 for
∗
CHAPTER 10 the bicyclo[2.2.1]heptyl, bicyclo[2.2.2]octyl, and adamantyl systems, respec-
Reactions Involving tively. Are the product trends consistent with these computational results,
Carbocations, Carbenes,
and Radicals as Reactive which presumably reflect the relative stability of the carbocation formed by the
Intermediates fragmentation?
pentane CH 2 Cl 2
[MeOH] R−Cl R−OCH 3 R−OH R−Cl R−OCH 3 R−OH
R = bicyclo[2.2.1]heptyl
0 100
0.25 15 3 82 64 trace 35
0.50 23 trace 77 59 1 40
1.00 45 trace 55 57 2 41
R = bicyclo[2.2.2]octyl
0 100
0.25 38 19 43 60 8 32
0.50 34 19 47 52 13 35
1.00 40 20 40 45 21 34
R = adamantyl
0 100
0.25 81 trace 19 93 trace 7
0.50 83 trace 17 91 trace 9
1.00 83 trace 17 79 10 11
10.21 a. The oxidation of norbornadiene by t-butyl perbenzoate and Cu(I) leads to
7-t-butoxynorbornadiene. Similarly, oxidation with dibenzoyl peroxide and
CuBr leads to 7-benzoyloxynorbornadiene. In both reactions, when a 2-
deuterated sample of norbornadiene is used, the deuterium is found distributed
among all positions in the product in approximately equal amounts. Provide
a mechanism that can account for this result.
b. A very direct synthesis of certain lactones involves heating an alkene with
a carboxylic acid and the Mn(III) salt of the acid. Suggest a mechanism by
which this reaction might occur.
CH (CH )
3
2 5
CH CO H
3
2
(CH ) CH CH + Mn(O CCH )
CH 3 2 5 2 2 3 3 O
O