Page 1028 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1028
1004 Direct nucleophilic displacement of halide and sulfonate groups from aromatic
rings is difficult, although the reaction can be useful in specific cases. These reactions
CHAPTER 11 can occur by either addition-elimination (Section 11.2.2) or elimination-addition
Aromatic Substitution (Section 11.2.3). Recently, there has been rapid development of metal ion catalysis, and
Reactions
old methods involving copper salts have been greatly improved. Palladium catalysts
for nucleophilic substitutions have been developed and have led to better procedures.
These reactions are discussed in Section 11.3.
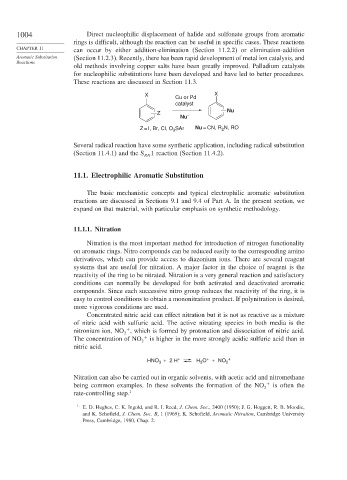
X X
Cu or Pd
catalyst
Z – Nu
Nu
Z = I, Br, Cl, O SAr Nu = CN, R N, RO
2
3
Several radical reaction have some synthetic application, including radical substitution
(Section 11.4.1) and the S RN 1 reaction (Section 11.4.2).
11.1. Electrophilic Aromatic Substitution
The basic mechanistic concepts and typical electrophilic aromatic substitution
reactions are discussed in Sections 9.1 and 9.4 of Part A. In the present section, we
expand on that material, with particular emphasis on synthetic methodology.
11.1.1. Nitration
Nitration is the most important method for introduction of nitrogen functionality
on aromatic rings. Nitro compounds can be reduced easily to the corresponding amino
derivatives, which can provide access to diazonium ions. There are several reagent
systems that are useful for nitration. A major factor in the choice of reagent is the
reactivity of the ring to be nitrated. Nitration is a very general reaction and satisfactory
conditions can normally be developed for both activated and deactivated aromatic
compounds. Since each successive nitro group reduces the reactivity of the ring, it is
easy to control conditions to obtain a mononitration product. If polynitration is desired,
more vigorous conditions are used.
Concentrated nitric acid can effect nitration but it is not as reactive as a mixture
of nitric acid with sulfuric acid. The active nitrating species in both media is the
nitronium ion, NO , which is formed by protonation and dissociation of nitric acid.
+
2
The concentration of NO + is higher in the more strongly acidic sulfuric acid than in
2
nitric acid.
+
HNO + 2 H + H O + NO 2 +
3
3
Nitration can also be carried out in organic solvents, with acetic acid and nitromethane
being common examples. In these solvents the formation of the NO 2 + is often the
rate-controlling step. 1
1
E. D. Hughes, C. K. Ingold, and R. I. Reed, J. Chem. Soc., 2400 (1950); J. G. Hoggett, R. B. Moodie,
and K. Schofield, J. Chem. Soc. B, 1 (1969); K. Schofield, Aromatic Nitration, Cambridge University
Press, Cambridge, 1980, Chap. 2.