Page 1032 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1032
1008 Scheme 11.1. (Continued)
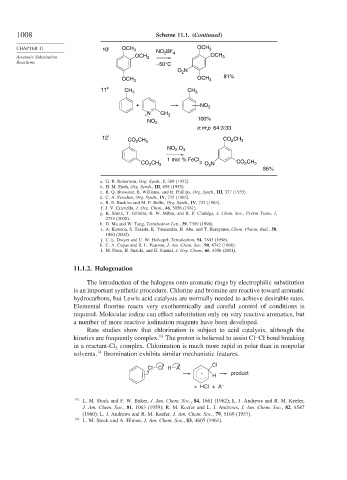
CHAPTER 11 10 j OCH 3 NO BF OCH 3
Aromatic Substitution OCH 3 2 4 OCH 3
Reactions –50°C
O N
2
OCH 3 OCH 3 81%
11 k CH 3 CH 3
+ NO 2
+ N CH 3
100%
NO 2
o:m:p 64:3:33
12 l CO CH 3 CO 2 CH 3
2
NO ,O
2
3
1 mol % FeCl
CO CH 3 3 O N CO CH 3
2
2
2
86%
a. G. R. Robertson, Org. Synth., I, 389 (1932).
b. H. M. Fitch, Org. Synth., III, 658 (1955).
c. R. Q. Brewster, B. Williams, and R. Phillips, Org. Synth., III, 337 (1955).
d. C. A. Fetscher, Org. Synth., IV, 735 (1963).
e. R. E. Buckles and M. P. Bellis, Org. Synth., IV, 722 (1963).
f. J. V. Crievello, J. Org. Chem., 46, 3056 (1981).
g. K. Smith, T. Gibbins, R. W. Millar, and R. P. Claridge, J. Chem. Soc., Perkin Trans. 1,
2753 (2000).
h. D. Ma and W. Tang, Tetrahedron Lett., 39, 7369 (1998).
i. A. Kawada, S. Takeda, K. Yamashita, H. Abe, and T. Harayama, Chem. Pharm. Bull., 50,
1060 (2002).
j. C. L. Dwyer and C. W. Holzapel, Tetrahedron, 54, 7843 (1998).
k. C. A. Cupas and R. L. Pearson, J. Am. Chem. Soc., 90, 4742 (1968).
l. M. Nose, H. Suzuki, and H. Suzuki, J. Org. Chem., 66, 4356 (2001).
11.1.2. Halogenation
The introduction of the halogens onto aromatic rings by electrophilic substitution
is an important synthetic procedure. Chlorine and bromine are reactive toward aromatic
hydrocarbons, but Lewis acid catalysts are normally needed to achieve desirable rates.
Elemental fluorine reacts very exothermically and careful control of conditions is
required. Molecular iodine can effect substitution only on very reactive aromatics, but
a number of more reactive iodination reagents have been developed.
Rate studies show that chlorination is subject to acid catalysis, although the
13
kinetics are frequently complex. The proton is believed to assist Cl–Cl bond breaking
in a reactant-Cl complex. Chlorination is much more rapid in polar than in nonpolar
2
solvents. 14 Bromination exhibits similar mechanistic features.
Cl
Cl Cl H A
+ product
H
+ HCl + A –
13 L. M. Stock and F. W. Baker, J. Am. Chem. Soc., 84, 1661 (1962); L. J. Andrews and R. M. Keefer,
J. Am. Chem. Soc., 81, 1063 (1959); R. M. Keefer and L. J. Andrews, J. Am. Chem. Soc., 82, 4547
(1960); L. J. Andrews and R. M. Keefer, J. Am. Chem. Soc., 79, 5169 (1957).
14
L. M. Stock and A. Himoe, J. Am. Chem. Soc., 83, 4605 (1961).