Page 1040 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1040
1016
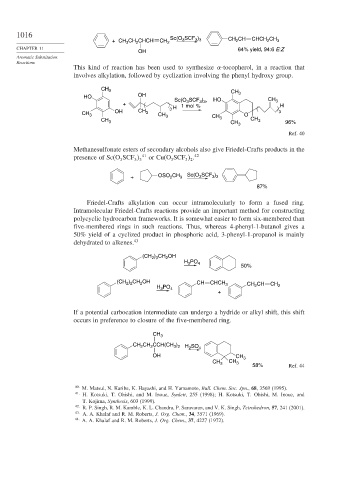
3
3 3
+ CH 3 CH 2 CHCH CH 2 Sc(O SCF ) CH CH CHCH CH 3
2
2
CHAPTER 11 64% yield, 94:6 E:Z
OH
Aromatic Substitution
Reactions
This kind of reaction has been used to synthesize -tocopherol, in a reaction that
involves alkylation, followed by cyclization involving the phenyl hydroxy group.
CH 3 CH
HO OH 3
Sc(O SCF ) , HO CH 3
3
+ H 1 mol % 3 3 H
CH 3 OH CH 3 CH 3 3 CH O 3
CH 3 3 CH 3 CH 3 96%
Ref. 40
Methanesulfonate esters of secondary alcohols also give Friedel-Crafts products in the
presence of Sc(O SCF 41 or Cu(O SCF . 42
3 3
3
3
3 2
Sc(O SCF )
+ OSO 2 CH 3 3 3 3
87%
Friedel-Crafts alkylation can occur intramolecularly to form a fused ring.
Intramolecular Friedel-Crafts reactions provide an important method for constructing
polycyclic hydrocarbon frameworks. It is somewhat easier to form six-membered than
five-membered rings in such reactions. Thus, whereas 4-phenyl-1-butanol gives a
50% yield of a cyclized product in phosphoric acid, 3-phenyl-1-propanol is mainly
dehydrated to alkenes. 43
) CH OH
(CH 2 3 2
H PO 4
3
50%
(CH ) CH OH CH CHCH 3 CH CH CH
2 2
2
H PO 4 2 2
3
+
If a potential carbocation intermediate can undergo a hydride or alkyl shift, this shift
occurs in preference to closure of the five-membered ring.
CH 3
CH CH CCH(CH ) 2 4
3 2 H SO
2
2
OH CH 3
CH CH 3
3
58% Ref. 44
40 M. Matsui, N. Karibe, K. Hayashi, and H. Yamamoto, Bull. Chem. Soc. Jpn., 68, 3569 (1995).
41 H. Kotsuki, T. Ohishi, and M. Inoue, Synlett, 255 (1998); H. Kotsuki, T. Ohishi, M. Inoue, and
T. Kojima, Synthesis, 603 (1999).
42
R. P. Singh, R. M. Kamble, K. L. Chandra, P. Saravaran, and V. K. Singh, Tetrahedron, 57, 241 (2001).
43 A. A. Khalaf and R. M. Roberts, J. Org. Chem., 34, 3571 (1969).
44
A. A. Khalaf and R. M. Roberts, J. Org. Chem., 37, 4227 (1972).