Page 1041 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1041
These results reflect a rather general tendency for 6 > 5, 7 in ring closure by 1017
intramolecular Friedel-Crafts reactions. 44 45 The difficulty in forming five-membered
rings may derive from steric and electronic factors. Some strain must develop because SECTION 11.1
2
of the sp carbons included in the ring. Perhaps more important is the need for approach Electrophilic Aromatic
Substitution
perpendicular to the ring. With three of the five carbons coplanar, it is difficult to align
the empty p orbital of the carbocation with the system.
+
H
Scheme 11.3 gives some examples of both inter- and intramolecular Friedel-Crafts
alkylations. Entry 1 is carried out using AlCl in an excess of refluxing benzene.
3
Entry 2 was also done using benzene as the solvent, but this reaction is done at 0 C.
A tertiary carbocation is generated by protonation of the double bond. Entry 3 involves
alkylation by both bromo substituents in the reactant. The reaction is carried out in
excess benzene, using AlBr . Entry 4 demonstrates the ability of a typical aromatic
3
sulfonic acid to generate a reactive carbocation by alkene protonation. The reaction
was carried out in excess toluene at 105 C. Note the relatively weak position selectivity
(see also Part A, Section 9.4.4). Secondary alkyl tosylates are also sources of reactive
carbocations under these conditions.
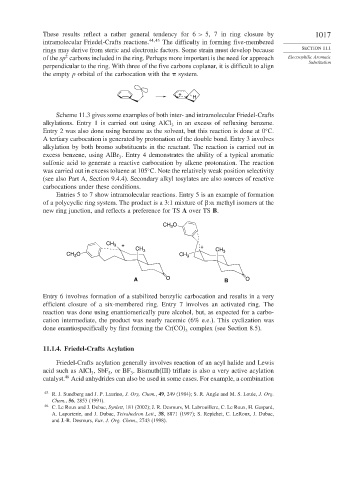
Entries 5 to 7 show intramolecular reactions. Entry 5 is an example of formation
of a polycyclic ring system. The product is a 3:1 mixture of : methyl isomers at the
new ring junction, and reflects a preference for TS A over TS B.
CH O
3
CH 3 + +
CH 3 CH
CH O CH 3 3
3
A O B O
Entry 6 involves formation of a stabilized benzylic carbocation and results in a very
efficient closure of a six-membered ring. Entry 7 involves an activated ring. The
reaction was done using enantiomerically pure alcohol, but, as expected for a carbo-
cation intermediate, the product was nearly racemic (6% e.e.). This cyclization was
done enantiospecifically by first forming the Cr(CO) complex (see Section 8.5).
3
11.1.4. Friedel-Crafts Acylation
Friedel-Crafts acylation generally involves reaction of an acyl halide and Lewis
acid such as AlCl , SbF ,orBF . Bismuth(III) triflate is also a very active acylation
3
3
5
46
catalyst. Acid anhydrides can also be used in some cases. For example, a combination
45 R. J. Sundberg and J. P. Laurino, J. Org. Chem., 49, 249 (1984); S. R. Angle and M. S. Louie, J. Org.
Chem., 56, 2853 (1991).
46
C. Le Roux and J. Dubac, Synlett, 181 (2002); J. R. Desmurs, M. Labrouillere, C. Le Roux, H. Gaspard,
A. Laporterie, and J. Dubac, Tetrahedron Lett., 38, 8871 (1997); S. Repichet, C. LeRoux, J. Dubac,
and J.-R. Desmurs, Eur. J. Org. Chem., 2743 (1998).