Page 1046 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1046
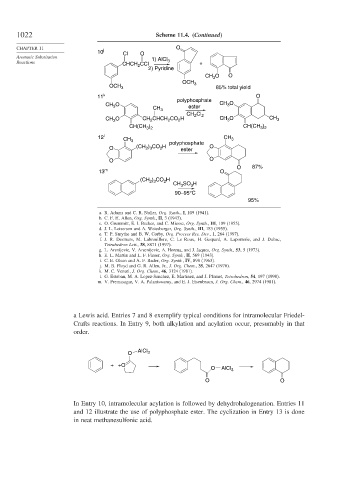
1022 Scheme 11.4. (Continued)
CHAPTER 11 O
10 j Cl O
Aromatic Substitution 1) AlCl
Reactions CHCH CCl 3 +
2
2) Pyridine
CH O O
3
OCH 3
OCH 3 85% total yield
11 k O
polyphosphate
CH O ester CH O
3
3
CH 3
Cl
CH 2 2
CH O CH CHCH CO H CH O CH 3
2
3
3
2
2
) CH(CH )
CH(CH 3 2 3 2
12 l CH 3 polyphosphate CH 3
O (CH ) CO H ester O
2
2 3
O O
O 87%
13 m O
) CO H
(CH 2 3 2
CH SO H
3
3
90–95°C
95%
a. R. Adams and C. R. Noller, Org. Synth., I, 109 (1941).
b. C. F. H. Allen, Org. Synth., II, 3 (1943).
c. O. Grummitt, E. I. Becker, and C. Miesse, Org. Synth., III, 109 (1955).
d. J. L. Leiserson and A. Weissberger, Org. Synth., III, 183 (1955).
e. T. P. Smythe and B. W. Corby, Org. Process Res. Dev., 1, 264 (1997).
f. J. R. Desmurs, M. Labrouillere, C. Le Roux, H. Gaspard, A. Laporterie, and J. Dubac,
Tetrahedron Lett., 38, 8871 (1997).
g. L. Arsnijevic, V. Arsenijevic, A. Horeua, and J. Jaques, Org. Synth., 53, 5 (1973).
h. E. L. Martin and L. F. Fieser, Org. Synth., II, 569 (1943).
i. C. E. Olson and A. F. Bader, Org. Synth., IV, 898 (1963).
j. M. B. Floyd and G. R. Allen, Jr., J. Org. Chem., 35, 2647 (1970).
k. M. C. Venuti, J. Org. Chem., 46, 3124 (1981).
l. G. Esteban, M. A. Lopez-Sanchez, E. Martinez, and J. Plumet, Tetrahedron, 54, 197 (1998).
m. V. Premasagar, V. A. Palaniswamy, and E. J. Eisenbraun, J. Org. Chem., 46, 2974 (1981).
a Lewis acid. Entries 7 and 8 exemplify typical conditions for intramolecular Friedel-
Crafts reactions. In Entry 9, both alkylation and acylation occur, presumably in that
order.
O AlCl 3
+ +O
O AlCl 3
O O
In Entry 10, intramolecular acylation is followed by dehydrohalogenation. Entries 11
and 12 illustrate the use of polyphosphate ester. The cyclization in Entry 13 is done
in neat methanesulfonic acid.