Page 1047 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1047
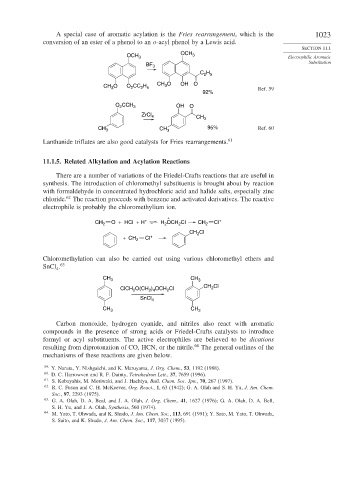
A special case of aromatic acylation is the Fries rearrangement, which is the 1023
conversion of an ester of a phenol to an o-acyl phenol by a Lewis acid.
SECTION 11.1
OCH 3 OCH 3 Electrophilic Aromatic
Substitution
BF 3
C H
2 5
CH O OH O
CH O O CC H 3 Ref. 59
2 5
2
3
92%
O CCH 3 OH O
2
ZrCl 4 CH 3
CH 3 CH 3 95% Ref. 60
Lanthanide triflates are also good catalysts for Fries rearrangements. 61
11.1.5. Related Alkylation and Acylation Reactions
There are a number of variations of the Friedel-Crafts reactions that are useful in
synthesis. The introduction of chloromethyl substituents is brought about by reaction
with formaldehyde in concentrated hydrochloric acid and halide salts, especially zinc
62
chloride. The reaction proceeds with benzene and activated derivatives. The reactive
electrophile is probably the chloromethylium ion.
+
CH 2 O + HCl + H + H OCH Cl CH 2 Cl +
2
2
CH Cl
2
+ CH 2 Cl +
Chloromethylation can also be carried out using various chloromethyl ethers and
SnCl . 63
4
CH 3 CH 3
CH Cl
O(CH ) OCH Cl 2
ClCH 2 2 4 2
SnCl 4
CH 3 CH 3
Carbon monoxide, hydrogen cyanide, and nitriles also react with aromatic
compounds in the presence of strong acids or Friedel-Crafts catalysts to introduce
formyl or acyl substituents. The active electrophiles are believed to be dications
resulting from diprotonation of CO, HCN, or the nitrile. 64 The general outlines of the
mechanisms of these reactions are given below.
59 Y. Naruta, Y. Nishgaichi, and K. Maruyama, J. Org. Chem., 53, 1192 (1988).
60 D. C. Harrowven and R. F. Dainty, Tetrahedron Lett., 37, 7659 (1996).
61
S. Kobayahis, M. Moriwaki, and J. Hachiya, Bull. Chem. Soc. Jpn., 70, 267 (1997).
62 R. C. Fuson and C. H. McKeever, Org. React., 1, 63 (1942); G. A. Olah and S. H. Yu, J. Am. Chem.
Soc., 97, 2293 (1975).
63 G. A. Olah, D. A. Beal, and J. A. Olah, J. Org. Chem., 41, 1627 (1976); G. A. Olah, D. A. Bell,
S. H. Yu, and J. A. Olah, Synthesis, 560 (1974).
64
M. Yato, T. Ohwada, and K. Shudo, J. Am. Chem. Soc., 113, 691 (1991); Y. Sato, M. Yato, T. Ohwada,
S. Saito, and K. Shudo, J. Am. Chem. Soc., 117, 3037 (1995).