Page 1049 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1049
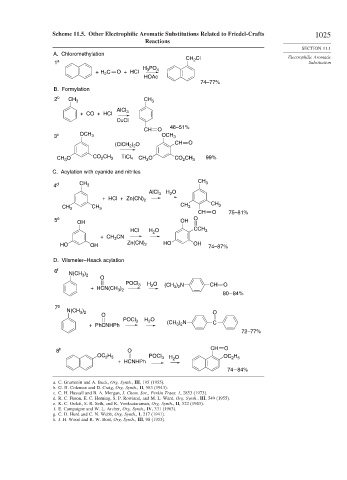
Scheme 11.5. Other Electrophilic Aromatic Substitutions Related to Friedel-Crafts 1025
Reactions
SECTION 11.1
A. Chloromethylation
CH Cl Electrophilic Aromatic
1 a 2 Substitution
H PO 4
3
+ H C O + HCl
2
HOAc
74–77%
B. Formylation
2 b CH 3 CH 3
AlCl 3
+ CO + HCl
CuCl
46–51%
CH O
3 c OCH 3 OCH 3
(ClCH ) O CH O
2 2
CH O CO CH 3 TiCl 4 CH O CO CH 3 99%
2
2
3
3
C. Acylation with cyanide and nitriles
CH 3
4 d CH 3
AlCl 3 H O
2
+ HCl + Zn(CN) 2
CH 3 CH 3 CH 3 CH 3
CH O 75–81%
5 e OH OH O
HCl H O CCH 3
2
+ CH CN
3
HO OH Zn(CN) 2 HO OH 74–87%
D. Vilsmeier–Haack acylation
6 f
N(CH 3 ) 2
O
POCl 3 H O (CH ) N CH O
+ HCN(CH ) 2 3 2
3 2
80 – 84%
7 g
)
N(CH 3 2 O
O
POCl 3 H O
2
3 2
+ PhCNHPh (CH ) N C
72–77%
8 h O CH O
H
H
OC 2 5 POCl 3 H O OC 2 5
2
+ HCNHPh
74 – 84%
a. C. Grummitt and A. Buck, Org. Synth., III, 195 (1955).
b. G. H. Coleman and D. Craig, Org. Synth., II, 583 (1943).
c. C. H. Hassall and B. A. Morgan, J. Chem. Soc., Perkin Trans. 1, 2853 (1973).
d. R. C. Fuson, E. C. Horning, S. P. Rowland, and M. L. Ward, Org. Synth., III, 549 (1955).
e. K. C. Gulati, S. R. Seth, and K. Venksataraman, Org. Synth., II, 522 (1943).
f. E. Campaigne and W. L. Archer, Org. Synth., IV, 331 (1963).
g. C. D. Hurd and C. N. Webb, Org. Synth., I, 217 (1941).
h. J. H. Wood and R. W. Bost, Org. Synth., III, 98 (1955).