Page 1058 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1058
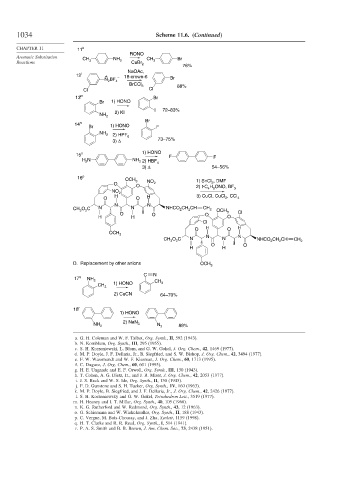
1034 Scheme 11.6. (Continued)
CHAPTER 11 11 k
RONO
Aromatic Substitution CH NH CH Br
Reactions 3 2 CuBr 3
2
76%
NaOAc,
12 l + 18-crown-6
BF – Br
N 2
4
BrCCl 3 88%
Cl Cl
13 m Br
Br 1) HONO
I 72–83%
2) KI
NH 2
Br
14 n
Br 1) HONO F
NH 2 2) HPF 6
3) Δ 73–75%
15 o 1) HONO F F
H N NH 2) HBF
2 2 4
3) Δ 54–56%
16 p OCH
2
O 3 NO 2 1) SnCl , DMF
O H ONO, BF
2) t-C 4 9 3
NO 2
H 3) CuCl, CuCl , CCl
O O H 2 4
N N
CH O C N N NHCO CH CH CH 2
2
2
3 2 OCH 3 Cl
O O O
H H O
Cl
O H O H
OCH
3 N N
CH O C N N NHCO CH CH CH 2
3
2
2
2
O O
H H
D. Replacement by other anions OCH 3
C N
17 q NH
2 1) HONO CH 3
CH 3
2) CuCN 64–70%
18 r
1) HONO
2) NaN
NH 2 3 N 3 88%
a. G. H. Coleman and W. F. Talbot, Org. Synth., II, 592 (1943).
b. N. Kornblum, Org. Synth., III, 295 (1955).
c. S. H. Korzeniowski, L. Blum, and G. W. Gokel, J. Org. Chem., 42, 1469 (1977).
d. M. P. Doyle, J. F. Dellaria, Jr., B. Siegfried, and S. W. Bishop, J. Org. Chem., 42, 3494 (1977).
e. F. W. Wassmundt and W. F. Kiesman, J. Org. Chem., 60, 1713 (1995).
f. C. Dugave, J. Org. Chem., 60, 601 (1995).
g. H. E. Ungnade and E. F. Orwoll, Org. Synth., III, 130 (1943).
h. T. Cohen, A. G. Dietz, Jr., and J. R. Miser, J. Org. Chem., 42, 2053 (1977).
i. J. S. Buck and W. S. Ide, Org. Synth., II, 130 (1943).
j. F. D. Gunstone and S. H. Tucker, Org. Synth., 1V, 160 (1963).
k. M. P. Doyle, B. Siegfried, and J. F. Dellaria, Jr., J. Org. Chem., 42, 2426 (1977).
l. S. H. Korzeniowsky and G. W. Gokel, Tetrahedron Lett., 3519 (1977).
m. H. Heaney and I. T. Millar, Org. Synth., 40, 105 (1960).
n. K. G. Rutherford and W. Redmond, Org. Synth., 43, 12 (1963).
o. G. Schiemann and W. Winkelmuller, Org. Synth., II, 188 (1943).
p. C. Vergne, M. Bois-Choussy, and J. Zhu, Synlett, 1159 (1998).
q. H. T. Clarke and R. R. Read, Org. Synth., I, 514 (1941).
r. P. A. S. Smith and B. B. Brown, J. Am. Chem. Soc., 73, 2438 (1951).