Page 1126 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1126
1102 illustrates the use of a piperidone salt for in situ generation of a dioxirane. The long
alkyl chain imparts phase transfer capability to the ketone. The dioxirane is generated
CHAPTER 12
in the aqueous phase but can carry out the epoxidation in the organic phase. Entries 3
Oxidations to 5 are examples of stereoselective epoxidations. In each case, high stereoselectivity
is observed in the presence of nearby functional groups. The exact origins of the
stereoselectivity are not clear.
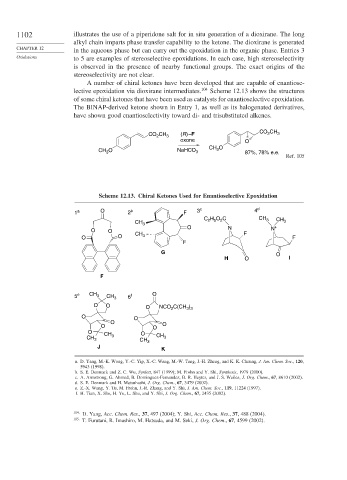
A number of chiral ketones have been developed that are capable of enantiose-
lective epoxidation via dioxirane intermediates. 104 Scheme 12.13 shows the structures
of some chiral ketones that have been used as catalysts for enantioselective epoxidation.
The BINAP-derived ketone shown in Entry 1, as well as its halogenated derivatives,
have shown good enantioselectivity toward di- and trisubstituted alkenes.
CO CH
CO CH 3 (R) –F 2 3
2
oxone O
CH O
CH O NaHCO 3 3 87%, 78% e.e.
3
Ref. 105
Scheme 12.13. Chiral Ketones Used for Enantioselective Epoxidation
1 a O 2 b F 3 c 4 d
H O C CH
C 2 5 2 3 CH
CH 3 3
O O O N F N +
O O CH 3 F
F
G O
H O I
F
5 e CH 3 CH 3 6 f O
O O O NCO 2 C(CH )
3 3
O O
O
O O O
O CH O
CH 3 3 CH 3
CH 3
J K
a. D. Yang, M.-K. Wong, Y.-C. Yip, X.-C. Wang, M.-W. Tang, J.-H. Zheng, and K. K. Cheung, J. Am. Chem. Soc., 120,
5943 (1998).
b. S. E. Denmark and Z. C. Wu, Synlett, 847 (1999); M. Frohn and Y. Shi, Synthesis, 1979 (2000).
c. A. Armstrong, G. Ahmed, B. Dominguez-Fernandez, B. R. Hayter, and J. S. Wailes, J. Org. Chem., 67, 8610 (2002).
d. S. E. Denmark and H. Matsuhashi, J. Org. Chem., 67, 3479 (2002).
e. Z.-X. Wang, Y. Tu, M. Frohn, J.-R. Zhang, and Y. Shi, J. Am. Chem. Soc., 119, 11224 (1997).
f. H. Tian, X. She, H. Yu, L. Shu, and Y. Shi, J. Org. Chem., 67, 2435 (2002).
104 D. Yang, Acc. Chem. Res., 37, 497 (2004); Y. Shi, Acc. Chem. Res., 37, 488 (2004).
105
T. Furutani, R. Imashiro, M. Hatsuda, and M. Seki, J. Org. Chem., 67, 4599 (2002).