Page 1127 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1127
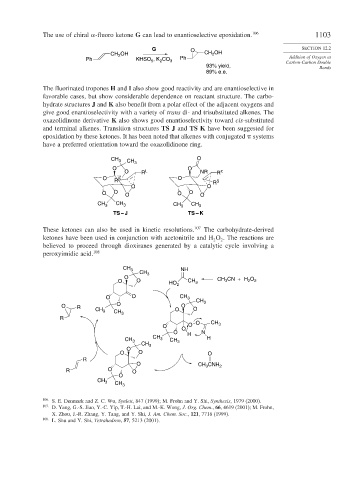
The use of chiral -fluoro ketone G can lead to enantioselective epoxidation. 106 1103
G O SECTION 12.2
CH OH CH 2 OH
2
Addition of Oxygen at
Ph KHSO , K CO 3 Ph Carbon-Carbon Double
2
5
93% yield, Bonds
89% e.e.
The fluorinated tropones H and I also show good reactivity and are enantioselective in
favorable cases, but show considerable dependence on reactant structure. The carbo-
hydrate structures J and K also benefit from a polar effect of the adjacent oxygens and
give good enantioselectivity with a variety of trans di- and trisubstituted alkenes. The
oxazolidinone derivative K also shows good enantioselectivity toward cis-substituted
and terminal alkenes. Transition structures TS J and TS K have been suggested for
epoxidation by these ketones. It has been noted that alkenes with conjugated systems
have a preferred orientation toward the oxazolidinone ring.
CH 3 CH 3 O
O O
O R L NR R π
O R S O S
O O R
O O O O O O
CH 3 CH 3 CH 3 CH 3
TS – J TS – K
These ketones can also be used in kinetic resolutions. 107 The carbohydrate-derived
ketones have been used in conjunction with acetonitrile and H O . The reactions are
2
2
believed to proceed through dioxiranes generated by a catalytic cycle involving a
peroxyimidic acid. 108
CH 3 NH
CH 3
O CH CN + H O
O O HO 2 CH 3 3 2 2
O O CH 3
CH 3
O
O R CH 3 CH O O O
R 3
O O O O CH 3
O H N
CH 3 CH 3 CH 3 H
CH 3
O
O O O
R
O CH CNH 2
3
R O O
O
CH 3
CH 3
106
S. E. Denmark and Z. C. Wu, Synlett, 847 (1999); M. Frohn and Y. Shi, Synthesis, 1979 (2000).
107 D. Yang, G.-S. Jiao, Y.-C. Yip, T.-H. Lai, and M.-K. Wong, J. Org. Chem., 66, 4619 (2001); M. Frohn,
X. Zhou, J.-R. Zhang, Y. Tang, and Y. Shi, J. Am. Chem. Soc., 121, 7718 (1999).
108
L. Shu and Y. Shi, Tetrahedron, 57, 5213 (2001).