Page 1131 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1131
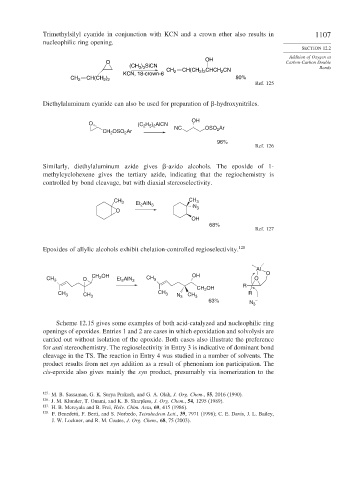
Trimethylsilyl cyanide in conjunction with KCN and a crown ether also results in 1107
nucleophilic ring opening.
SECTION 12.2
Addition of Oxygen at
OH
O Carbon-Carbon Double
(CH 3 ) 3 SiCN Bonds
CH 2 CH(CH ) CHCH CN
2
2 2
KCN, 18-crown-6
CH 2 CH(CH ) 80%
2 2
Ref. 125
Diethylaluminum cyanide can also be used for preparation of
-hydroxynitriles.
OH
O (C H ) AlCN NC OSO 2 Ar
2 5 2
CH OSO 2 Ar
2
96%
Ref. 126
Similarly, diethylaluminum azide gives
-azido alcohols. The epoxide of 1-
methylcyclohexene gives the tertiary azide, indicating that the regiochemistry is
controlled by bond cleavage, but with diaxial stereoselectivity.
CH 3 Et AlN 3 CH 3
2
O N 3
OH
68%
Ref. 127
Epoxides of allylic alcohols exhibit chelation-controlled regioselectivity. 128
Al
O
CH OH OH
CH 3 O 2 Et AlN 3 CH 3 O
2
R
CH OH
2
CH 3 CH 3 CH 3 N 3 CH 3 R
63% N 3 –
Scheme 12.15 gives some examples of both acid-catalyzed and nucleophilic ring
openings of epoxides. Entries 1 and 2 are cases in which epoxidation and solvolysis are
carried out without isolation of the epoxide. Both cases also illustrate the preference
for anti stereochemistry. The regioselectivity in Entry 3 is indicative of dominant bond
cleavage in the TS. The reaction in Entry 4 was studied in a number of solvents. The
product results from net syn addition as a result of phenonium ion participation. The
cis-epoxide also gives mainly the syn product, presumably via isomerization to the
125
M. B. Sassaman, G. K. Surya Prakash, and G. A. Olah, J. Org. Chem., 55, 2016 (1990).
126
J. M. Klunder, T. Onami, and K. B. Sharpless, J. Org. Chem., 54, 1295 (1989).
127 H. B. Mereyala and B. Frei, Helv. Chim. Acta, 69, 415 (1986).
128
F. Benedetti, F. Berti, and S. Norbedo, Tetrahedron Lett., 39, 7971 (1998); C. E. Davis, J. L. Bailey,
J. W. Lockner, and R. M. Coates, J. Org. Chem., 68, 75 (2003).