Page 1132 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1132
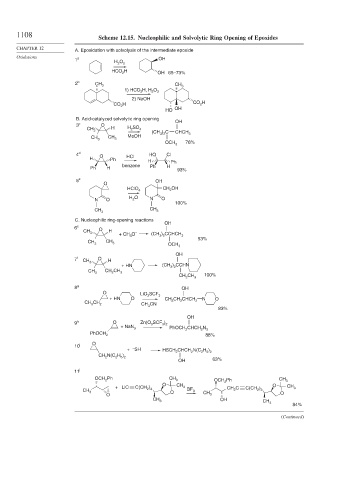
1108 Scheme 12.15. Nucleophilic and Solvolytic Ring Opening of Epoxides
CHAPTER 12
A. Epoxidation with solvolysis of the intermediate epoxide
Oxidations a OH
1
H O 2
2
HCO H OH 65–73%
2
2 b CH 3 CH 3
1) HCO H, H O
2 2 2
2) NaOH
CO H
CO H 2
2
HO OH
B. Acid-catalyzed solvolytic ring opening OH
3 c O
CH H H 2 SO 4
3
(CH ) C CHCH
CH CH MeOH 3 2 3
3 3
76%
OCH 3
4 d O HCl HO Cl
H Ph H Ph
benzene Ph H
Ph H
93%
5 e OH
O
OH
HClO 4 CH 2
H O
N O 2 N O
100%
CH CH 3
3
C. Nucleophilic ring-opening reactions
OH
6 c O
CH 3 H + CH O – (CH 3 2 3
) CCHCH
3
CH 3 CH 3 OCH 3 53%
OH
7 f CH 3 O H
+ HN (CH ) CCHN
3 2
CH 3 CH CH 3
2
CH CH 100%
2 3
8 g OH
O SCF
LiO 3 3
+ HN O CH CH CHCH N O
CH 3 2 2
CH 3 2 CH CN
3
83%
OH
9 h O Zn(O SCF )
3 2
3
+ NaN 3 PhOCH CHCH N
PhOCH 2 2 3
2 88%
10 i O
–
+ SH HSCH CHCH N(C H )
2 2 2 5 2
N(C H )
2 5 2 63%
CH 2
OH
11 j
OCH Ph CH 3 OCH 2 Ph CH 3
2
O CH
+ LiC C(CH ) 3 C C(CH ) O CH 3
2 3 BF CH 2 2 3
CH 3 O 3 O
O CH 3
OH
CH 3 CH
3
84%
(Continued)