Page 1133 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1133
Scheme 12.15. (Continued) 1109
a. A. Roebuck and H. Adkins, Org. Synth., III, 217 (1955). SECTION 12.2
b. T. R. Kelly, J. Org. Chem., 37, 3393 (1972).
c. S. Winstein and L. L. Ingraham, J. Am. Chem. Soc., 74, 1160 (1952). Addition of Oxygen at
d. G. Berti, F. Bottari, P. L. Ferrarini, and B. Macchia, J. Org. Chem., 30, 4091 (1965). Carbon-Carbon Double
e. M. L. Rueppel and H. Rapoport, J. Am. Chem. Soc., 94, 3877 (1972). Bonds
f. T. Colclough, J. I. Cunneen, and C. G. Moore, Tetrahedron, 15, 187 (1961).
g. J. Auge and F. Leroy, Tetrahedron Lett., 37, 7715 (1996).
h. M. Chini, P. Crotti, and F. Macchia, Tetrahedron Lett., 31, 5641 (1990).
i. D. M. Burness and H. O. Bayer, J. Org. Chem., 28, 2283 (1963).
j. Z. Liu, C. Yu, R.-F.Wang, and G. Li, Tetrahedron Lett., 39, 5261 (1998).
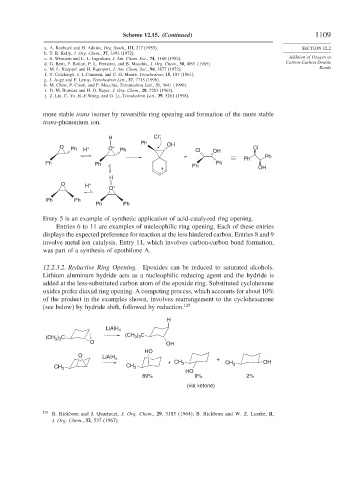
more stable trans isomer by reversible ring opening and formation of the more stable
trans-phenonium ion.
H Cl –
Ph
O Ph H + O + Ph OH Cl OH Cl
Ph
Ph
Ph Ph Ph Ph
+ OH
H
O H +
O +
Ph Ph
Ph Ph
Entry 5 is an example of synthetic application of acid-catalyzed ring opening.
Entries 6 to 11 are examples of nucleophilic ring opening. Each of these entries
displays the expected preference for reaction at the less hindered carbon. Entries 8 and 9
involve metal ion catalysis. Entry 11, which involves carbon-carbon bond formation,
was part of a synthesis of epothilone A.
12.2.3.2. Reductive Ring Opening. Epoxides can be reduced to saturated alcohols.
Lithium aluminum hydride acts as a nucleophilic reducing agent and the hydride is
added at the less-substituted carbon atom of the epoxide ring. Substituted cyclohexene
oxides prefer diaxial ring opening. A competing process, which accounts for about 10%
of the product in the examples shown, involves rearrangement to the cyclohexanone
(see below) by hydride shift, followed by reduction. 129
H
LiAlH 4
(CH ) C
) C
(CH 3 3 3 3
O OH
HO
O LiAlH 4 +
+ CH 3 CH 3 OH
CH 3 CH 3
HO
89% 9% 2%
(via ketone)
129
B. Rickborn and J. Quartucci, J. Org. Chem., 29, 3185 (1964); B. Rickborn and W. Z. Lamke, II,
J. Org. Chem., 32, 537 (1967).