Page 1138 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1138
1114
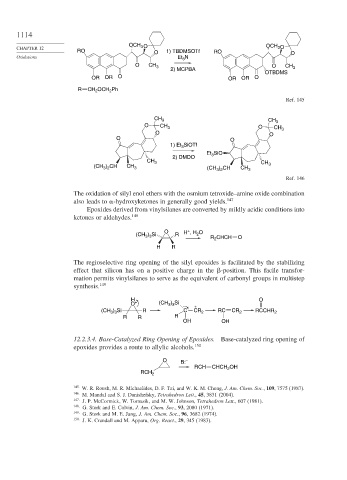
OCH 3 O OCH O
CHAPTER 12 3
RO O 1) TBDMSOTf RO O
Oxidations Et 3 N
O CH 3 O CH 3
2) MCPBA
OTBDMS
OR OR O OR OR O
R CH OCH Ph
2
2
Ref. 145
CH 3 CH 3
O CH 3 O CH 3
O O
O O
1) Et SiOTf
3
Et SiO
2) DMDO 3
CH 3 CH
(CH ) CH CH 3 (CH ) CH CH 3 3
3 2
3 2
Ref. 146
The oxidation of silyl enol ethers with the osmium tetroxide–amine oxide combination
also leads to -hydroxyketones in generally good yields. 147
Epoxides derived from vinylsilanes are converted by mildly acidic conditions into
ketones or aldehydes. 148
O H , H O
+
(CH 3 ) 3 Si R 2
R 2 CHCH O
H R
The regioselective ring opening of the silyl epoxides is facilitated by the stabilizing
effect that silicon has on a positive charge in the
-position. This facile transfor-
mation permits vinylsilanes to serve as the equivalent of carbonyl groups in multistep
synthesis. 149
H O
O + (CH ) Si
3 3
+
) Si R C CR RC CR RCCHR
(CH 3 3 2 2 2
R R R
OH OH
12.2.3.4. Base-Catalyzed Ring Opening of Epoxides. Base-catalyzed ring opening of
epoxides provides a route to allylic alcohols. 150
O B: –
RCH CHCH OH
2
RCH 2
145
W. R. Roush, M. R. Michaelides, D. F. Tai, and W. K. M. Chong, J. Am. Chem. Soc., 109, 7575 (1987).
146 M. Mandal and S. J. Danishefsky, Tetrahedron Lett., 45, 3831 (2004).
147
J. P. McCormick, W. Tomasik, and M. W. Johnson, Tetrahedron Lett., 607 (1981).
148 G. Stork and E. Colvin, J. Am. Chem. Soc., 93, 2080 (1971).
149 G. Stork and M. E. Jung, J. Am. Chem. Soc., 96, 3682 (1974).
150
J. K. Crandall and M. Apparu, Org. React., 29, 345 (1983).