Page 1139 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1139
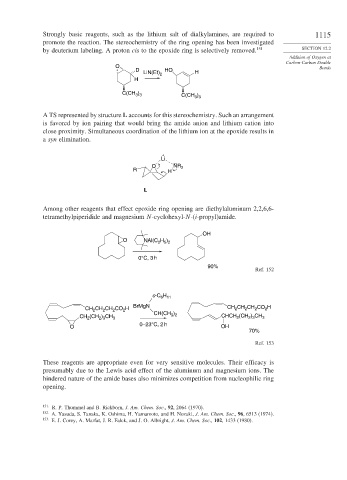
Strongly basic reagents, such as the lithium salt of dialkylamines, are required to 1115
promote the reaction. The stereochemistry of the ring opening has been investigated
by deuterium labeling. A proton cis to the epoxide ring is selectively removed. 151 SECTION 12.2
Addition of Oxygen at
Carbon-Carbon Double
O Bonds
D HO
LiN(Et) 2 H
H
)
C(CH )
C(CH 3 3
3 3
A TS represented by structure L accounts for this stereochemistry. Such an arrangement
is favored by ion pairing that would bring the amide anion and lithium cation into
close proximity. Simultaneous coordination of the lithium ion at the epoxide results in
a syn elimination.
+
Li _
O NR 2
R H
L
Among other reagents that effect epoxide ring opening are diethylaluminum 2,2,6,6-
tetramethylpiperidide and magnesium N-cyclohexyl-N-(i-propyl)amide.
OH
O NAl(C 2 5 2
H )
0°C, 3 h
90%
Ref. 152
c-C H
6 11
CH CH CO H BrMgN CH CH CH CO H
CH 2 2 2 2 2 2 2 2
3 2
CH (CH ) CH 3 CH(CH ) CHCH 2 (CH 2 ) 3 CH 3
2
2 3
0–23°C, 2 h
O OH
70%
Ref. 153
These reagents are appropriate even for very sensitive molecules. Their efficacy is
presumably due to the Lewis acid effect of the aluminum and magnesium ions. The
hindered nature of the amide bases also minimizes competition from nucleophilic ring
opening.
151 R. P. Thummel and B. Rickborn, J. Am. Chem. Soc., 92, 2064 (1970).
152 A. Yasuda, S. Tanaka, K. Oshima, H. Yamamoto, and H. Nozaki, J. Am. Chem. Soc., 96, 6513 (1974).
153
E. J. Corey, A. Marfat, J. R. Falck, and J. O. Albright, J. Am. Chem. Soc., 102, 1433 (1980).