Page 1143 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1143
Singlet oxygen decays to the ground state triplet at a rate that is strongly dependent 1119
on the solvent. 162 Measured half-lives range from about 700 s in carbon tetrachloride
to 2 s in water. The choice of solvent can therefore have a pronounced effect on the SECTION 12.3
efficiency of oxidation; the longer the singlet state lifetime, the more likely it is that Allylic Oxidation
reaction with the alkene can occur.
The reactivity order of alkenes is that expected for attack by an electrophilic
reagent. Reactivity increases with the number of alkyl substituents. 163 Terminal alkenes
‡
are relatively inert. The reaction has a low H and relative reactivity is dominated
by entropic factors. 164 Steric effects govern the direction of approach of the oxygen,
so the hydroperoxy group is usually introduced on the less hindered face of the double
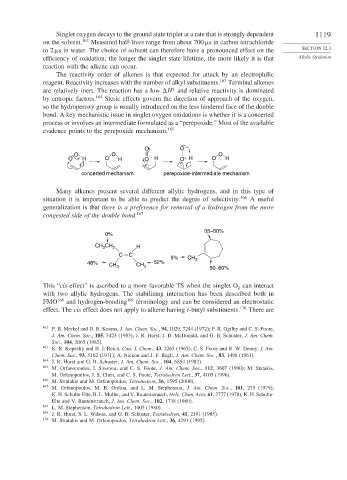
bond. A key mechanistic issue in singlet oxygen oxidations is whether it is a concerted
process or involves an intermediate formulated as a “perepoxide.” Most of the available
evidence points to the perepoxide mechanism. 165
O O –
O O O
O H O H O H O + H O H
concerted mechanism perepoxide-intermediate mechanism
Many alkenes present several different allylic hydrogens, and in this type of
situation it is important to be able to predict the degree of selectivity. 166 A useful
generalization is that there is a preference for removal of a hydrogen from the more
congested side of the double bond. 167
35–50%
0%
CH CH 2 H
3
C C
5% CH 3
48% CH 3 CH 3 52% 50–60%
This “cis effect” is ascribed to a more favorable TS when the singlet O can interact
2
with two allylic hydrogens. The stabilizing interaction has been described both in
FMO 168 and hydrogen-bonding 169 terminology and can be considered an electrostatic
effect. The cis effect does not apply to alkene having t-butyl substituents. 170 There are
162 P. B. Merkel and D. R. Kearns, J. Am. Chem. Soc., 94, 1029, 7244 (1972); P. R. Ogilby and C. S. Foote,
J. Am. Chem. Soc., 105, 3423 (1983); J. R. Hurst, J. D. McDonald, and G. B. Schuster, J. Am. Chem.
Soc., 104, 2065 (1982).
163 K. R. Kopecky and H. J. Reich, Can. J. Chem., 43, 2265 (1965); C. S. Foote and R. W. Denny, J. Am.
Chem. Soc., 93, 5162 (1971); A. Nickon and J. F. Bagli, J. Am. Chem. Soc., 83, 1498 (1961).
164
J. R. Hurst and G. B. Schuster, J. Am. Chem. Soc., 104, 6854 (1982).
165 M. Orfanopoulos, I. Smonou, and C. S. Foote, J. Am. Chem. Soc., 112, 3607 (1990); M. Statakis,
M. Orfanopoulos, J. S. Chen, and C. S. Foote, Tetrahedron Lett., 37, 4105 (1996).
166 M. Stratakis and M. Orfanopoulos, Tetrahedron, 56, 1595 (2000).
167
M. Orfanopoulos, M. B. Grdina, and L. M. Stephenson, J. Am. Chem. Soc., 101, 275 (1979);
K. H. Schulte-Elte, B. L. Muller, and V. Rautenstrauch, Helv. Chim. Acta, 61, 2777 (1978); K. H. Schulte-
Elte and V. Rautenstrauch, J. Am. Chem. Soc., 102, 1738 (1980).
168 L. M. Stephenson, Tetrahedron Lett., 1005 (1980).
169 J. R. Hurst, S. L. Wilson, and G. B. Schuster, Tetrahedron, 41, 2191 (1985).
170
M. Stratakis and M. Orfanopoulos, Tetrahedron Lett., 36, 4291 (1995).