Page 1147 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1147
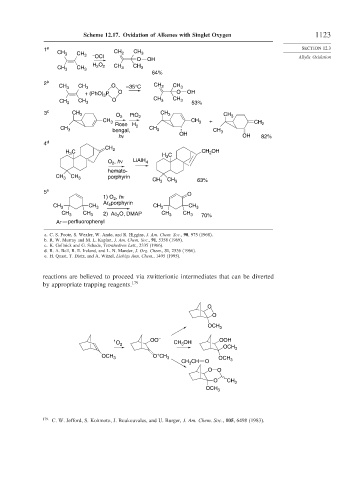
Scheme 12.17. Oxidation of Alkenes with Singlet Oxygen 1123
1 a SECTION 12.3
CH 3 CH 3 – CH 2 CH 3
OCl
O OH Allylic Oxidation
H O CH CH
CH 2 2 3 3
CH 3 3
64%
2 b
CH 3 CH 3 O –35°C CH 2 CH 3
P O O OH
+ (PhO) 3
CH O CH 3 CH 3
CH 3 3 53%
3 c CH 3 O 2 PtO CH 3 CH 3
CH 3 2 CH 3 + CH 2
Rose
CH 3 bengal, H 2 CH 3 CH 3
hν OH OH 82%
4 d
CH
C 2 CH OH
H 3 2
H C
3
O , hν LiAlH 4
2
hemato-
CH 3 CH 3 porphyrin CH 3 CH 3 63%
5 e
1) O , hν O
2
Ar porphyrin
CH 3 CH 3 4 CH 3 CH 3
CH 3 CH 3 2) Ac 2 O, DMAP CH 3 CH 3 70%
Ar perfluorophenyl
a. C. S. Foote, S. Wexler, W. Ando, and R. Higgins, J. Am. Chem. Soc., 90, 975 (1968).
b. R. W. Murray and M. L. Kaplan, J. Am. Chem. Soc., 91, 5358 (1969).
c. K. Gollnick and G. Schade, Tetrahedron Lett., 2335 (1966).
d. R. A. Bell, R. E. Ireland, and L. N. Mander, J. Org. Chem., 31, 2536 (1966).
e. H. Quast, T. Dietz, and A. Witzel, Liebigs Ann. Chem., 1495 (1995).
reactions are believed to proceed via zwitterionic intermediates that can be diverted
by appropriate trapping reagents. 179
O
O
OCH 3
1 O OO – CH 3 OH OOH
2
OCH 3
+
OCH 3 O CH 3 OCH
CH CH O 3
3
O O
O CH 3
OCH 3
179
C. W. Jefford, S. Kohmoto, J. Boukouvalas, and U. Burger, J. Am. Chem. Soc., 105, 6498 (1983).