Page 1148 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1148
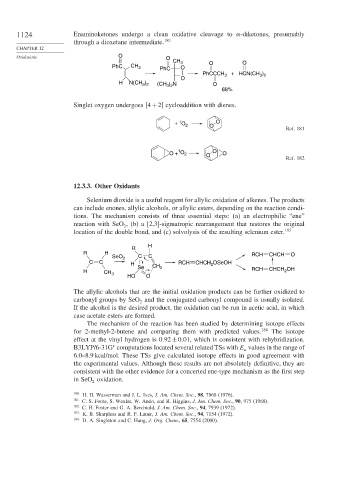
1124 Enaminoketones undergo a clean oxidative cleavage to -diketones, presumably
through a dioxetane intermediate. 180
CHAPTER 12
Oxidations O O
CH 3 O O
PhC CH 3 PhC O
+ HCN(CH )
PhCCCH 3 3 2
O
)
H N(CH 3 2 (CH ) N O
3 2
68%
Singlet oxygen undergoes 4+2 cycloaddition with dienes.
1
+ O O
2 O
Ref. 181
1
O + O 2 O O O
Ref. 182
12.3.3. Other Oxidants
Selenium dioxide is a useful reagent for allylic oxidation of alkenes. The products
can include enones, allylic alcohols, or allylic esters, depending on the reaction condi-
tions. The mechanism consists of three essential steps: (a) an electrophilic “ene”
reaction with SeO , (b) a [2,3]-sigmatropic rearrangement that restores the original
2
location of the double bond, and (c) solvolysis of the resulting selenium ester. 183
H
R
R H RCH CHCH O
SeO 2 C C
C C H RCH CHCH 2 OSeOH
Se CH 2 RCH OH
H CH 3 CHCH 2
HO O
The allylic alcohols that are the initial oxidation products can be further oxidized to
carbonyl groups by SeO and the conjugated carbonyl compound is usually isolated.
2
If the alcohol is the desired product, the oxidation can be run in acetic acid, in which
case acetate esters are formed.
The mechanism of the reaction has been studied by determining isotope effects
for 2-methyl-2-butene and comparing them with predicted values. 184 The isotope
effect at the vinyl hydrogen is 0 92 ± 0 01, which is consistent with rehybridization.
B3LYP/6-31G computations located several related TSs with E values in the range of
∗
a
6.0–8.9 kcal/mol. These TSs give calculated isotope effects in good agreement with
the experimental values. Although these results are not absolutely definitive, they are
consistent with the other evidence for a concerted ene-type mechanism as the first step
in SeO oxidation.
2
180 H. H. Wasserman and J. L. Ives, J. Am. Chem. Soc., 98, 7868 (1976).
181
C. S. Foote, S. Wexler, W. Ando, and R. Higgins, J. Am. Chem. Soc., 90, 975 (1968).
182
C. H. Foster and G. A. Berchtold, J. Am. Chem. Soc., 94, 7939 (1972).
183 K. B. Sharpless and R. F. Lauer, J. Am. Chem. Soc., 94, 7154 (1972).
184
D. A. Singleton and C. Hang, J. Org. Chem., 65, 7554 (2000).