Page 1149 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1149
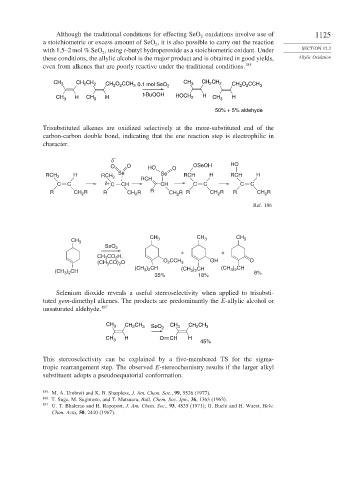
Although the traditional conditions for effecting SeO oxidations involve use of 1125
2
a stoichiometric or excess amount of SeO , it is also possible to carry out the reaction
2
with 1.5–2 mol % SeO , using t-butyl hydroperoxide as a stoichiometric oxidant. Under SECTION 12.3
2
these conditions, the allylic alcohol is the major product and is obtained in good yields, Allylic Oxidation
even from alkenes that are poorly reactive under the traditional conditions. 185
CH CH CH CH CH
CH 3 2 2 CH 2 O CCH 0.1 mol SeO 2 3 2 2 CH O CCH 3
2
2
3
2
t-BuOOH HOCH H
H CH H 2 CH H
CH 3 3 3
50% + 5% aldehyde
Trisubstituted alkenes are oxidized selectively at the more-substituted end of the
carbon-carbon double bond, indicating that the ene reaction step is electrophilic in
character.
δ –
O O HO O OSeOH HO
RCH 2 H RCH 2 Se Se RCH H RCH H
RCH
C C δ+ C CH CH C C C C
R CH 2 R R CH 2 R R CH R R CH R R CH R
2
2
2
Ref. 186
CH CH CH
CH 3 3 3 3
SeO 2
+ +
CH CO H,
3
2
(CH CO) O O 2 CCH 3 OH O
2
3
) CH
) CH
(CH 3 2 (CH ) CH (CH 3 2
(CH ) CH 3 2 8%
3 2
35% 18%
Selenium dioxide reveals a useful stereoselectivity when applied to trisubsti-
tuted gem-dimethyl alkenes. The products are predominantly the E-allylic alcohol or
unsaturated aldehyde. 187
CH 3 CH 2 CH 3 SeO 2 CH 3 CH 2 CH 3
CH 3 H O CH H 45%
This stereoselectivity can be explained by a five-membered TS for the sigma-
tropic rearrangement step. The observed E-stereochemistry results if the larger alkyl
substituent adopts a pseudoequatorial conformation.
185
M. A. Umbreit and K. B. Sharpless, J. Am. Chem. Soc., 99, 5526 (1977).
186 T. Suga, M. Sugimoto, and T. Matsuura, Bull. Chem. Soc. Jpn., 36, 1363 (1963).
187
U. T. Bhalerao and H. Rapoport, J. Am. Chem. Soc., 93, 4835 (1971); G. Buchi and H. Wuest, Helv.
Chim. Acta, 50, 2440 (1967).