Page 1160 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1160
1136 O O
O F
O O
CHAPTER 12 +
(CH 3 ) 3 C F
Oxidations
F C(CH 3 ) 3 (CH 3 ) 3 C 29%
71%
O
O
O F O
O
F + F
(CH 3 ) 3 C
(CH 3 ) 3 C
9% C(CH 3 ) 3 91% O
R
O R R O O R O
O O
O O O : :
O O O
: : : : : O : F H
O H O H F H H
H H H H
H
H F H H H H
F H Migration occurs
This conformation
disfavored by dipole- mainly through this
No strong conformational bias conformation
in trans isomer. Both groups dipole repulsion
migrate to a similar extent.
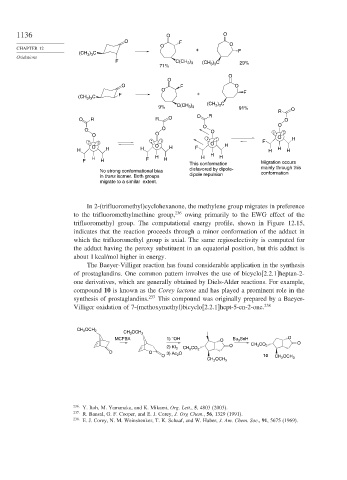
In 2-(trifluoromethyl)cyclohexanone, the methylene group migrates in preference
to the trifluoromethylmethine group, 236 owing primarily to the EWG effect of the
trifluoromethyl group. The computational energy profile, shown in Figure 12.15,
indicates that the reaction proceeds through a minor conformation of the adduct in
which the trifluoromethyl group is axial. The same regioselectivity is computed for
the adduct having the peroxy substituent in an equatorial position, but this adduct is
about 1 kcal/mol higher in energy.
The Baeyer-Villiger reaction has found considerable application in the synthesis
of prostaglandins. One common pattern involves the use of bicyclo[2.2.1]heptan-2-
one derivatives, which are generally obtained by Diels-Alder reactions. For example,
compound 10 is known as the Corey lactone and has played a prominent role in the
synthesis of prostaglandins. 237 This compound was originally prepared by a Baeyer-
Villiger oxidation of 7-(methoxymethyl)bicyclo[2.2.1]hept-5-en-2-one. 238
CH 3 OCH 2
CH 3 OCH 2 I
–
MCPBA 1) OH Bu 3 SnH O
O O
O CH 3 CO 2
2) KI 3 CH 3 CO 2
O O 3) Ac 2 O
O 10 CH 2 OCH 3
CH 2 OCH 3
236
Y. Itoh, M. Yamanaka, and K. Mikami, Org. Lett., 5, 4803 (2003).
237 R. Bansal, G. F. Cooper, and E. J. Corey, J. Org Chem., 56, 1329 (1991).
238
E. J. Corey, N. M. Weinshenker, T. K. Schaaf, and W. Huber, J. Am. Chem. Soc., 91, 5675 (1969).