Page 1162 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1162
1138 O CF 3 O CF 3
O CH 3 CO 3 H O
CHAPTER 12 O + regioisomeric
CH 3 CO 2 H, NaOAc
Oxidations lactone
20°C
OTBDMS OTBDMS
steps
HO
CO 2 CH(CH 3 ) 3
CF 3
O
HO
OH
Travopros
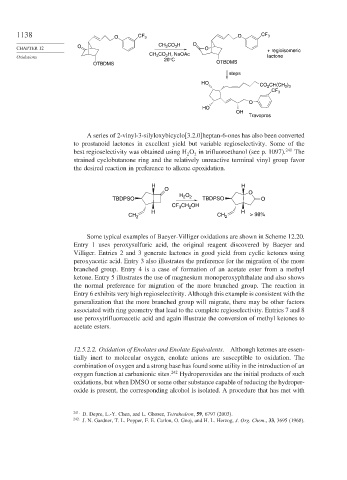
A series of 2-vinyl-3-silyloxybicyclo[3.2.0]heptan-6-ones has also been converted
to prostanoid lactones in excellent yield but variable regioselectivity. Some of the
best regioselectivity was obtained using H O in trifluoroethanol (see p. 1097). 241 The
2
2
strained cyclobutanone ring and the relatively unreactive terminal vinyl group favor
the desired reaction in preference to alkene epoxidation.
H H
O
O
H O 2
2
TBDPSO TBDPSO O
CF CH OH
3
2
H H
CH 2 CH 2 > 98%
Some typical examples of Baeyer-Villiger oxidations are shown in Scheme 12.20.
Entry 1 uses peroxysulfuric acid, the original reagent discovered by Baeyer and
Villiger. Entries 2 and 3 generate lactones in good yield from cyclic ketones using
peroxyacetic acid. Entry 3 also illustrates the preference for the migration of the more
branched group. Entry 4 is a case of formation of an acetate ester from a methyl
ketone. Entry 5 illustrates the use of magnesium monoperoxyphthalate and also shows
the normal preference for migration of the more branched group. The reaction in
Entry 6 exhibits very high regioselectivity. Although this example is consistent with the
generalization that the more branched group will migrate, there may be other factors
associated with ring geometry that lead to the complete regioselectivity. Entries 7 and 8
use peroxytrifluoroacetic acid and again illustrate the conversion of methyl ketones to
acetate esters.
12.5.2.2. Oxidation of Enolates and Enolate Equivalents. Although ketones are essen-
tially inert to molecular oxygen, enolate anions are susceptible to oxidation. The
combination of oxygen and a strong base has found some utility in the introduction of an
oxygen function at carbanionic sites. 242 Hydroperoxides are the initial products of such
oxidations, but when DMSO or some other substance capable of reducing the hydroper-
oxide is present, the corresponding alcohol is isolated. A procedure that has met with
241 D. Depre, L.-Y. Chen, and L. Ghosez, Tetrahedron, 59, 6797 (2003).
242
J. N. Gardner, T. L. Popper, F. E. Carlon, O. Gnoj, and H. L. Herzog, J. Org. Chem., 33, 3695 (1968).