Page 1165 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1165
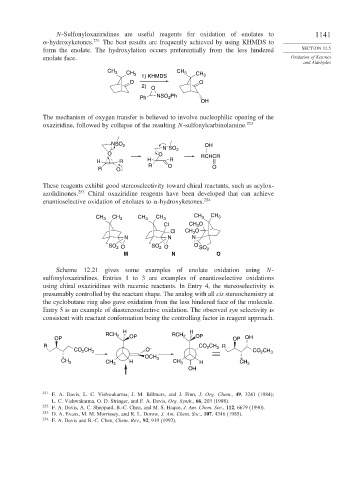
N-Sulfonyloxaziridines are useful reagents for oxidation of enolates to 1141
-hydroxyketones. 251 The best results are frequently achieved by using KHMDS to
form the enolate. The hydroxylation occurs preferentially from the less hindered SECTION 12.5
enolate face. Oxidation of Ketones
and Aldehydes
CH 3 CH 3 1) KHMDS CH 3 CH 3
O O
2) O
Ph NSO Ph
2
OH
The mechanism of oxygen transfer is believed to involve nucleophilic opening of the
oxaziridine, followed by collapse of the resulting N-sulfonylcarbinolamine. 252
NSO 2 OH
–
N SO 2
O O RCHCR
H R H R
R O O
R O–
These reagents exhibit good stereoselectivity toward chiral reactants, such as acylox-
azolidinones. 253 Chiral oxaziridine reagents have been developed that can achieve
enantioselective oxidation of enolates to -hydroxyketones. 254
CH 3 CH 3 CH 3 CH 3 CH 3 CH 3
Cl CH O
3
Cl CH O
3
N N N
SO 2 O SO 2 O O SO 2
M N O
Scheme 12.21 gives some examples of enolate oxidation using N-
sulfonyloxaziridines. Entries 1 to 3 are examples of enantioselective oxidations
using chiral oxaziridines with racemic reactants. In Entry 4, the stereoselectivity is
presumably controlled by the reactant shape. The analog with all cis stereochemistry at
the cyclobutane ring also gave oxidation from the less hindered face of the molecule.
Entry 5 is an example of diastereoselective oxidation. The observed syn selectivity is
consistent with reactant conformation being the controlling factor in reagent approach.
H H
RCH RCH
OP 2 OP 2 OP OP OH
R CO CH 3 R
2
CO CH 3 O – CO 2 CH 3
2
OCH 3
CH 3 CH 3 H CH 3 H CH 3
OH
251
F. A. Davis, L. C. Vishwakarma, J. M. Billmers, and J. Finn, J. Org. Chem., 49, 3241 (1984);
L. C. Vishwakarma, O. D. Stringer, and F. A. Davis, Org. Synth., 66, 203 (1988).
252 F. A. Davis, A. C. Sheppard, B.-C. Chen, and M. S. Haque, J. Am. Chem. Soc., 112, 6679 (1990).
253 D. A. Evans, M. M. Morrissey, and R. L. Dorow, J. Am. Chem. Soc., 107, 4346 (1985).
254
F. A. Davis and B.-C. Chen, Chem. Rev., 92, 919 (1992).