Page 1166 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1166
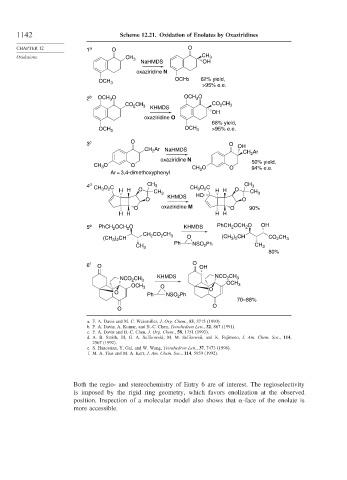
1142 Scheme 12.21. Oxidation of Enolates by Oxaziridines
CHAPTER 12 1 a O O
Oxidations CH 3 CH 3
NaHMDS OH
oxaziridine N
OCH 3 OCH3 62% yield,
>95% e.e.
2 b OCH O OCH 3 O
3
CO CH 3 KHMDS CO 2 CH 3
2
OH
oxaziridine O
68% yield,
OCH 3 OCH 3 >95% e.e.
3 c O O OH
CH Ar NaHMDS CH Ar
2
2
oxaziridine N
O 50% yield,
CH 3 O O O
CH 3 94% e.e.
Ar = 3,4-dimethoxyphenyl
4 d CH O C H H O CH CH CH 3 O C H H O CH CH 3
3
3
2
3
2
O 3 KHMDS HO O
O oxaziridine M O 90%
H H H H
5 e PhCH OCH O KHMDS PhCH OCH O OH
2
2
2
2
CO CH
CH 2 2 3 (CH ) CH
(CH ) CH O 3 2 CO CH 3
2
3 2
CH 3 Ph NSO Ph CH 3
2
80%
6 f O O OH
NCO CH 3 KHMDS NCO CH 3
2
2
OCH
O 3
OCH 3
O O
Ph NSO Ph
2
70–88%
O
O
a. F. A. Davis and M. C. Weismiller, J. Org. Chem., 55, 3715 (1990).
b. F. A. Davis, A. Kumar, and B.-C. Chen, Tetrahedron Lett., 32, 867 (1991).
c. F. A. Davis and B.-C. Chen, J. Org. Chem., 58, 1751 (1993).
d. A. B. Smith, III, G. A. Sulikowski, M. M. Sulikowsii, and K. Fujimoto, J. Am. Chem. Soc., 114,
2567 (1992).
e. S. Hanessian, Y. Gai, and W. Wang, Tetrahedron Lett., 37, 7473 (1996).
f. M. A. Tius and M. A. Kerr, J. Am. Chem. Soc., 114, 5959 (1992).
Both the regio- and stereochemistry of Entry 6 are of interest. The regioselectivity
is imposed by the rigid ring geometry, which favors enolization at the observed
position. Inspection of a molecular model also shows that -face of the enolate is
more accessible.