Page 1172 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1172
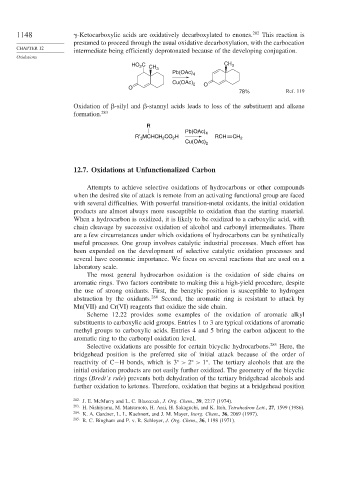
1148 -Ketocarboxylic acids are oxidatively decarboxylated to enones. 282 This reaction is
presumed to proceed through the usual oxidative decarboxylation, with the carbocation
CHAPTER 12
intermediate being efficiently deprotonated because of the developing conjugation.
Oxidations
HO C CH CH 3
2
3
Pb(OAc) 4
Cu(OAc) 2
O O
78% Ref. 119
Oxidation of
-silyl and
-stannyl acids leads to loss of the substituent and alkene
formation. 283
R
Pb(OAc) 4
R′ MCHCH CO H RCH CH 2
2
2
3
Cu(OAc) 2
12.7. Oxidations at Unfunctionalized Carbon
Attempts to achieve selective oxidations of hydrocarbons or other compounds
when the desired site of attack is remote from an activating functional group are faced
with several difficulties. With powerful transition-metal oxidants, the initial oxidation
products are almost always more susceptible to oxidation than the starting material.
When a hydrocarbon is oxidized, it is likely to be oxidized to a carboxylic acid, with
chain cleavage by successive oxidation of alcohol and carbonyl intermediates. There
are a few circumstances under which oxidations of hydrocarbons can be synthetically
useful processes. One group involves catalytic industrial processes. Much effort has
been expended on the development of selective catalytic oxidation processes and
several have economic importance. We focus on several reactions that are used on a
laboratory scale.
The most general hydrocarbon oxidation is the oxidation of side chains on
aromatic rings. Two factors contribute to making this a high-yield procedure, despite
the use of strong oxidants. First, the benzylic position is susceptible to hydrogen
abstraction by the oxidants. 284 Second, the aromatic ring is resistant to attack by
Mn(VII) and Cr(VI) reagents that oxidize the side chain.
Scheme 12.22 provides some examples of the oxidation of aromatic alkyl
substituents to carboxylic acid groups. Entries 1 to 3 are typical oxidations of aromatic
methyl groups to carboxylic acids. Entries 4 and 5 bring the carbon adjacent to the
aromatic ring to the carbonyl oxidation level.
Selective oxidations are possible for certain bicyclic hydrocarbons. 285 Here, the
bridgehead position is the preferred site of initial attack because of the order of
reactivity of C−H bonds, which is 3 > 2 > 1 . The tertiary alcohols that are the
initial oxidation products are not easily further oxidized. The geometry of the bicyclic
rings (Bredt’s rule) prevents both dehydration of the tertiary bridgehead alcohols and
further oxidation to ketones. Therefore, oxidation that begins at a bridgehead position
282
J. E. McMurry and L. C. Blaszczak, J. Org. Chem., 39, 2217 (1974).
283 H. Nishiyama, M. Matsumoto, H. Arai, H. Sakaguchi, and K. Itoh, Tetrahedron Lett., 27, 1599 (1986).
284 K. A. Gardner, L. L. Kuehnert, and J. M. Mayer, Inorg. Chem., 36, 2069 (1997).
285
R. C. Bingham and P. v. R. Schleyer, J. Org. Chem., 36, 1198 (1971).