Page 1178 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1178
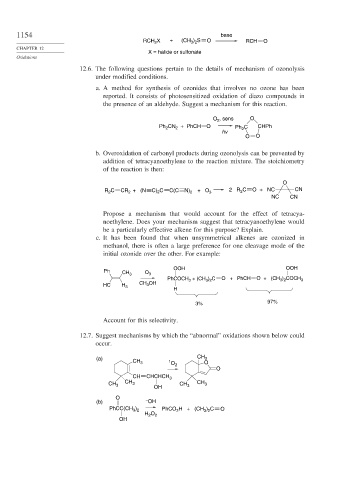
1154 base
) S
RCH X + (CH 3 2 O RCH O
2
CHAPTER 12
X = halide or sulfonate
Oxidations
12.6. The following questions pertain to the details of mechanism of ozonolysis
under modified conditions.
a. A method for synthesis of ozonides that involves no ozone has been
reported. It consists of photosensitized oxidation of diazo compounds in
the presence of an aldehyde. Suggest a mechanism for this reaction.
O , sens O
2
Ph CN + PhCH O Ph C CHPh
2
2
2
hv
O O
b. Overoxidation of carbonyl products during ozonolysis can be prevented by
addition of tetracyanoethylene to the reaction mixture. The stoichiometry
of the reaction is then:
O
R C CR + (N C) 2 C C(C N) 2 +O 3 2 R 2 C O + NC CN
2
2
NC CN
Propose a mechanism that would account for the effect of tetracya-
noethylene. Does your mechanism suggest that tetracyanoethylene would
be a particularly effective alkene for this purpose? Explain.
c. It has been found that when unsymmetrical alkenes are ozonized in
methanol, there is often a large preference for one cleavage mode of the
initial ozonide over the other. For example:
OOH OOH
Ph
CH 3 O 3
PhCOCH 3 + (CH 3 ) 2 C O + PhCH O + (CH 3 ) 2 COCH 3
CH 3 OH
HC H 3
H
3% 97%
Account for this selectivity.
12.7. Suggest mechanisms by which the “abnormal” oxidations shown below could
occur.
(a) CH 3 1 O CH O
3
2
O
CH CHCHCH 3
CH 3 CH 3 OH CH 3 CH 3
O
(b) – OH
PhCC(CH ) PhCO H + (CH ) C O
3 2
3 2
2
H 2 O 2
OH