Page 1182 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1182
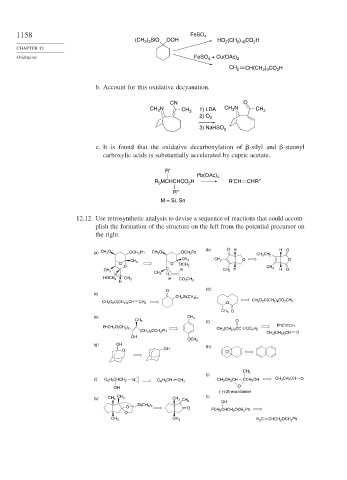
1158 FeSO 4
(CH ) SiO OOH HO 2 (CH 2 ) 10 CO 2 H
3 3
CHAPTER 12
Oxidations FeSO + Cu(OAc) 2
4
) CO H
CH 2 CH(CH 2 3 2
b. Account for this oxidative decyanation.
CN O
CH N CH 3 1) LDA CH 3 N CH 3
3
2) O
2
3) NaHSO 3
c. It is found that the oxidative decarboxylation of
-silyl and
-stannyl
carboxylic acids is substantially accelerated by cupric acetate.
R′
Pb(OAc) 4
R MCHCHCO H R′CH CHR″
3
2
R″
M = Si, Sn
12.12. Use retrosynthetic analysis to devise a sequence of reactions that could accom-
plish the formation of the structure on the left from the potential precursor on
the right.
(b) O H H O
(a) CH 3 O OCH 2 Ph CH 3 O OCH 2 Ph
CH 3 CH 2
CH 3 CH 3 O O
CH 3
O O OCH 3
O C CH 3
CH 3 H CH 3 H H O
H CH 3 H
HOCH 2 CH 3 H CO 2 CH 3
H
(d)
O
(c)
CH 2 Si(CH 3 ) 3
CH 3 O 2 C(CH 2 ) 4 CH CH 2 O CH 3 O 2 C(CH 2 ) 4 CO 2 CH 3
CH 3 O
(e) CH 3
CH 3 (f) O
PhC CH.
PhCH 2 O(CH 2 ) 2 CH 3 (CH 2 ) 3 CC CC 6 H 5
(CH 2 ) 2 OCH 2 Ph
CH 3 (CH 2 ) 3 CH O
OH
OCH 3
(g) OH (h)
O OH
O
CH 3
(j)
(i) C 6 H 5 CHCH 2 N C 6 H 5 CH CH 2 CH 3 CH 2 CH CCH 2 OH CH 3 CH 2 CH O
OH O
(-)-(S)-enantiomer
(l)
(k) CH 3 CH 3 CH 3 CH 2
OH
O Si(CH 3 ) 3 O
O FCH 2 CHCH 2 OCH 2 Ph
CH 3 CH 3 H 2 C CHCH 2 OCH 2 Ph