Page 1190 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1190
1166 groups are considered. It is frequently necessary to interconvert functional groups,
which may be to done to develop a particular kind of reactivity at a center or to avoid
CHAPTER 13 interference with a reaction step. Protective groups and synthetic equivalent groups are
Multistep Syntheses important for planning of functional group transformations. Owing to the large number
of procedures for interconverting the common functional groups, achieving the final
array of functionality is often less difficult than establishing the overall molecular
skeleton and stereochemistry.
The synthetic plan must also provide for control of stereochemistry. In the case of
cyclic compounds, advantage often can be taken of the facial preferences of the rings
and the stereoselectivity of reagents to establish the stereochemistry of substituents.
For example, the syn-directive effect of hydroxy groups in epoxidation (see p. 1093)
or the strong preference for anti addition in iodolactonization (see p. 311) can be
used to determine the configuration of new stereogenic centers. Similarly, the cyclic
TS of sigmatropic rearrangements often allows predictable stereoselectivity. Chiral
auxiliaries and catalysts provide means of establishing configuration in enantioselective
syntheses. A plan for a stereo- or enantioselective synthesis must include the basis for
controlling the configuration at each stereocenter.
The care with which a synthesis is analyzed and planned will have a great impact
on the likelihood of its success. The investment of material and effort that is made
when the synthesis is begun may be lost if the plan is faulty. Even with the best
of planning, however, unexpected problems are often encountered. This circumstance
again tests the ingenuity of the chemist to devise a modified plan that can overcome
the unanticipated obstacle.
13.1.2. Synthetic Equivalent Groups
Retrosynthetic analysis may identify a need to use synthetic equivalent groups.
These groups are synthons that correspond structurally to a subunit of the target
structure, but in which the reactivity of the functionality is masked or modified. As an
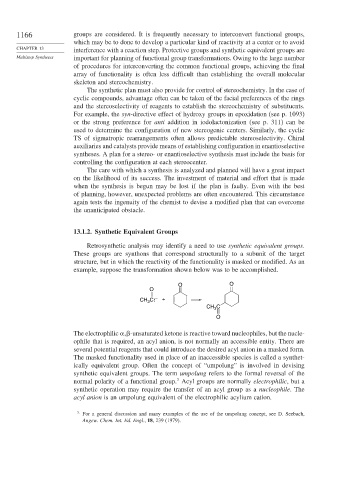
example, suppose the transformation shown below was to be accomplished.
O O
O
CH C: – +
3
CH C
3
O
The electrophilic -unsaturated ketone is reactive toward nucleophiles, but the nucle-
ophile that is required, an acyl anion, is not normally an accessible entity. There are
several potential reagents that could introduce the desired acyl anion in a masked form.
The masked functionality used in place of an inaccessible species is called a synthet-
ically equivalent group. Often the concept of “umpolung” is involved in devising
synthetic equivalent groups. The term umpolung refers to the formal reversal of the
3
normal polarity of a functional group. Acyl groups are normally electrophilic, but a
synthetic operation may require the transfer of an acyl group as a nucleophile. The
acyl anion is an umpolung equivalent of the electrophilic acylium cation.
3
For a general discussion and many examples of the use of the umpolung concept, see D. Seebach,
Angew. Chem. Int. Ed. Engl., 18, 239 (1979).