Page 624 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 624
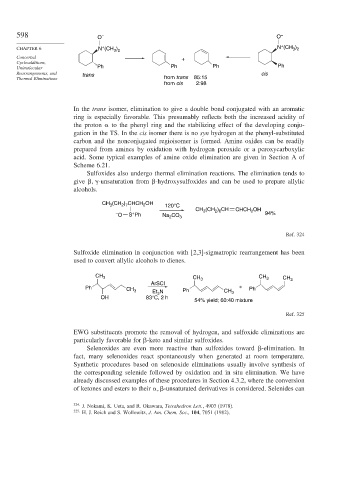
598 O – O –
+
+
CHAPTER 6 N (CH ) N (CH )
3 2
3 2
Concerted +
Cycloadditions,
Unimolecular Ph Ph Ph Ph
Rearrangements, and trans cis
Thermal Eliminations from trans 85:15
from cis 2:98
In the trans isomer, elimination to give a double bond conjugated with an aromatic
ring is especially favorable. This presumably reflects both the increased acidity of
the proton to the phenyl ring and the stabilizing effect of the developing conju-
gation in the TS. In the cis isomer there is no syn hydrogen at the phenyl-substituted
carbon and the nonconjugated regioisomer is formed. Amine oxides can be readily
prepared from amines by oxidation with hydrogen peroxide or a peroxycarboxylic
acid. Some typical examples of amine oxide elimination are given in Section A of
Scheme 6.21.
Sulfoxides also undergo thermal elimination reactions. The elimination tends to
give -unsaturation from -hydroxysulfoxides and can be used to prepare allylic
alcohols.
CH (CH ) CHCH OH 120°C
2 7
2
3
CH (CH ) CH CHCH OH
3
2 6
+
– O S Ph Na CO 3 2 94%
2
Ref. 324
Sulfoxide elimination in conjunction with [2,3]-sigmatropic rearrangement has been
used to convert allylic alcohols to dienes.
CH 3 CH 3 CH 3 CH 3
ArSCl
Ph CH 3 Et N Ph CH 3 + Ph
3
OH 83°C, 2 h
54% yield; 60:40 mixture
Ref. 325
EWG substituents promote the removal of hydrogen, and sulfoxide eliminations are
particularly favorable for -keto and similar sulfoxides.
Selenoxides are even more reactive than sulfoxides toward -elimination. In
fact, many selenoxides react spontaneously when generated at room temperature.
Synthetic procedures based on selenoxide eliminations usually involve synthesis of
the corresponding selenide followed by oxidation and in situ elimination. We have
already discussed examples of these procedures in Section 4.3.2, where the conversion
of ketones and esters to their -unsaturated derivatives is considered. Selenides can
324 J. Nokami, K. Ueta, and R. Okawara, Tetrahedron Lett., 4903 (1978).
325
H. J. Reich and S. Wollowitz, J. Am. Chem. Soc., 104, 7051 (1982).