Page 627 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 627
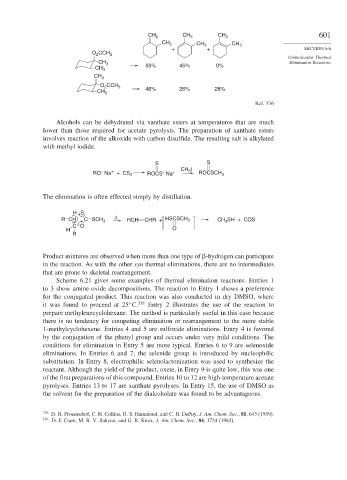
CH 2 CH 3 CH 3 601
CH 2 CH 3 CH 3
+ + SECTION 6.6
O CCH 3
2
Unimolecular Thermal
CH 3 Elimination Reactions
CH 3 55% 45% 0%
CH 3
O CCH 3
2
CH 3 46% 26% 28%
Ref. 336
Alcohols can be dehydrated via xanthate esters at temperatures that are much
lower than those required for acetate pyrolysis. The preparation of xanthate esters
involves reaction of the alkoxide with carbon disulfide. The resulting salt is alkylated
with methyl iodide.
S S
+
–
RO Na + CS 2 ROCS Na + CH 3 I ROCSCH 3
–
The elimination is often effected simply by distillation.
H S
R CH C SCH 3 Δ RCH CHR + HSCSCH 3 CH SH + COS
3
CO
H O
R
Product mixtures are observed when more than one type of -hydrogen can participate
in the reaction. As with the other syn thermal eliminations, there are no intermediates
that are prone to skeletal rearrangement.
Scheme 6.21 gives some examples of thermal elimination reactions. Entries 1
to 3 show amine-oxide decompositions. The reaction in Entry 1 shows a preference
for the conjugated product. This reaction was also conducted in dry DMSO, where
it was found to proceed at 25 C. 338 Entry 2 illustrates the use of the reaction to
prepare methylenecyclohexane. The method is particularly useful in this case because
there is no tendency for competing elimination or rearrangement to the more stable
1-methylcyclohexene. Entries 4 and 5 are sulfoxide eliminations. Entry 4 is favored
by the conjugation of the phenyl group and occurs under very mild conditions. The
conditions for elimination in Entry 5 are more typical. Entries 6 to 9 are selenoxide
eliminations. In Entries 6 and 7, the selenide group is introduced by nucleophilic
substitution. In Entry 8, electrophilic selenolactonization was used to synthesize the
reactant. Although the yield of the product, oxete, in Entry 9 is quite low, this was one
of the first preparations of this compound. Entries 10 to 12 are high-temperature acetate
pyrolyses. Entries 13 to 17 are xanthate pyrolyses. In Entry 15, the use of DMSO as
the solvent for the preparation of the dialcoholate was found to be advantageous.
336 D. H. Froemsdorf, C. H. Collins, G. S. Hammond, and C. H. DePuy, J. Am. Chem. Soc., 81, 643 (1959).
338
D. J. Cram, M. R. V. Sahyun, and G. R. Knox, J. Am. Chem. Soc., 84, 1734 (1962).