Page 628 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 628
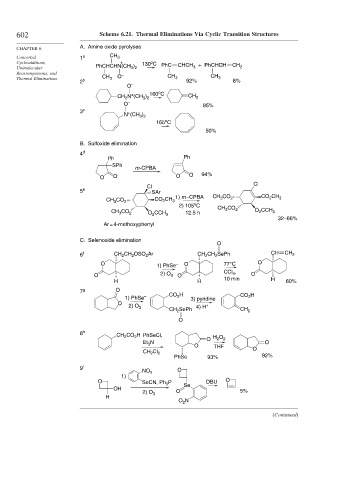
602 Scheme 6.21. Thermal Eliminations Via Cyclic Transition Structures
CHAPTER 6 A. Amine oxide pyrolyses
Concerted 1 a CH 3
o
Cycloadditions, + 130 C PhC CHCH + PhCHCH CH
3 2
Unimolecular PhCHCHN(CH ) 3 2
Rearrangements, and –
Thermal Eliminations CH 3 O CH 3 CH 3
2 b 92% 8%
O –
o
+
CH N (CH ) 160 C CH 2
2
3 2
O – 85%
3 c +
N (CH )
3 2
o
165 C
50%
B. Sulfoxide elimination
4 d
Ph Ph
SPh
m -CPBA
O O O O 94%
Cl
Cl
5 e SAr
2
CH CO 2 CO CH 3 1) m –CPBA CH 3 CO 2 CO CH 3
2
3
o
2) 105 C CH CO
CH CO 2 O CCH 3 12.5 h 3 2 O CCH 3
2
3
2
32–66%
Ar = 4-methoxyphenyl
C. Selenoxide elimination
O
6 f CH 2 CH OSO Ar CH CH SePh CH CH 2
2
2
2
2
O 1) PhSe – O 77°C O
O 2) O O CCl 4 , O
3
H H 10 min H 60%
7 g O
2
2
1) PhSe – CO H 3) pyridine CO H
O
+
2) O 4) H
3
CH SePh CH 2
2
O
8 h CH CO H PhSeCl, H O
2
2
2
Et N O O THF 2 O
3
CH Cl 2 O
2
PhSe 93% 92%
9 i O
NO 2
1)
O SeCN, Ph P Se DBU O
3
OH O 5%
2) O 3
H
O N
2
(Continued)