Page 787 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 787
750 Carraher’s Polymer Chemistry
3
H C CH 3 H 3 C H C
3
Cl Cl Cl C
Cl Cl Cl Cl
H C H C H C H C
3
3
3
3
1. 2. 3. 4.
Cl Cl Cl CH 3 H C Cl
3
H C CH 3
3
Cl Cl
Cl Cl
C CH CH
H 3
CH 3 3 3
1. 2. 3. 4.
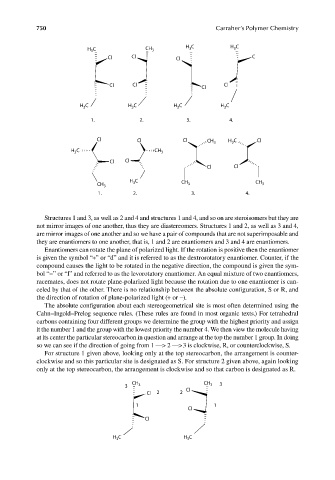
Structures 1 and 3, as well as 2 and 4 and structures 1 and 4, and so on are steroisomers but they are
not mirror images of one another, thus they are diastereomers. Structures 1 and 2, as well as 3 and 4,
are mirror images of one another and so we have a pair of compounds that are not superimposable and
they are enantiomers to one another, that is, 1 and 2 are enantiomers and 3 and 4 are enantiomers.
Enantiomers can rotate the plane of polarized light. If the rotation is positive then the enantiomer
is given the symbol “+” or “d” and it is referred to as the dextrorotatory enantiomer. Counter, if the
compound causes the light to be rotated in the negative direction, the compound is given the sym-
bol “−” or “l” and referred to as the levorotatory enantiomer. An equal mixture of two enantiomers,
racemates, does not rotate plane-polarized light because the rotation due to one enantiomer is can-
celed by that of the other. There is no relationship between the absolute configuration, S or R, and
the direction of rotation of plane-polarized light (+ or −).
The absolute configuration about each stereogeometrical site is most often determined using the
Cahn–Ingold–Prelog sequence rules. (These rules are found in most organic texts.) For tetrahedral
carbons containing four different groups we determine the group with the highest priority and assign
it the number 1 and the group with the lowest priority the number 4. We then view the molecule having
at its center the particular stereocarbon in question and arrange at the top the number 1 group. In doing
so we can see if the direction of going from 1 —> 2 —> 3 is clockwise, R, or counterclockwise, S.
For structure 1 given above, looking only at the top stereocarbon, the arrangement is counter-
clockwise and so this particular site is designated as S. For structure 2 given above, again looking
only at the top stereocarbon, the arrangement is clockwise and so that carbon is designated as R.
CH CH 3
3 3 3
Cl 2 2 Cl
1 1
Cl
Cl
C C
H 3 H 3
9/14/2010 3:44:49 PM
K10478.indb 750
K10478.indb 750 9/14/2010 3:44:49 PM