Page 788 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 788
Stereogeometry of Polymers 751
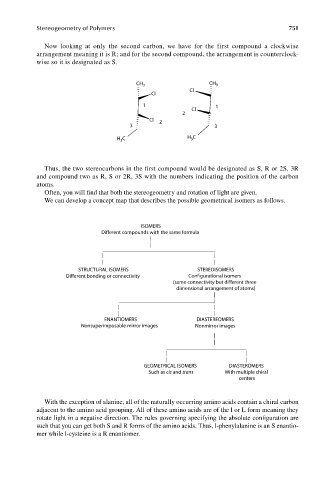
Now looking at only the second carbon, we have for the first compound a clockwise
arrangement meaning it is R; and for the second compound, the arrangement is counterclock-
wise so it is designated as S.
CH 3 CH 3
Cl
Cl
1 1
Cl
2
Cl 2
3 3
C H 3 C
H 3
Thus, the two stereocarbons in the first compound would be designated as S, R or 2S, 3R
and compound two as R, S or 2R, 3S with the numbers indicating the position of the carbon
atoms.
Often, you will find that both the stereogeometry and rotation of light are given.
We can develop a concept map that describes the possible geometrical isomers as follows.
ISOMERS
Different compounds with the same formula
|
|
| |
| |
STRUCTURAL ISOMERS STEREOISOMERS
Different bonding or connectivity Configurational isomers
(same connectivity but different three
dimensional arrangement of atoms)
|
|
| |
| |
ENANTIOMERS DIASTEREOMERS
Nonsuperimposable mirror images Nonmirror images
|
|
| |
| |
GEOMETRICAL ISOMERS DIASTEROMERS
Such as cis and trans With multiple chiral
centers
With the exception of alanine, all of the naturally occurring amino acids contain a chiral carbon
adjacent to the amino acid grouping. All of these amino acids are of the l or L form meaning they
rotate light in a negative direction. The rules governing specifying the absolute confi guration are
such that you can get both S and R forms of the amino acids. Thus, l-phenylalanine is an S enantio-
mer while l-cysteine is a R enantiomer.
9/14/2010 3:44:49 PM
K10478.indb 751 9/14/2010 3:44:49 PM
K10478.indb 751