Page 505 - Elements of Chemical Reaction Engineering Ebook
P. 505
4 76 Steady-State Nonisothermal Reactor Design Chap. 8
500 350 400 450 500 800
T
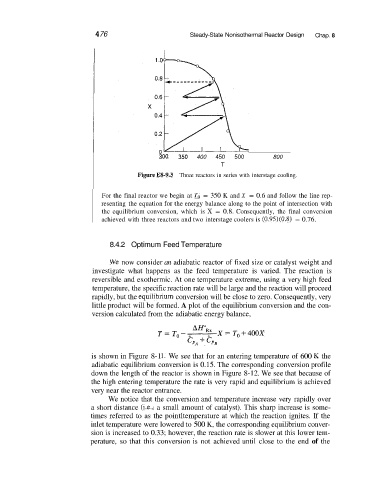
Figure E8-9.3 Three reactors in series with interstage cooling.
For the final reactor we begin at To = 350 K and X = 0.6 and follow the line rep-
resenting the equation for the energy balance along to the point of intersection with
the equilibrium conversion, which is X = 0.8. Consequently, the final conversion
achieved with three reactors and two interstage coolers is (0.95)(0.8) = 0.76.
8.4.2 Optimum Feed Temperature
We now consider an adiabatic reactor of fixed size or catalyst weight and
investigate what happens as the feed temperature is varied. The reaction is
reversible and exothermic. At one temperature extreme, using a very high feed
temperature, the specific reaction rate will be large and the reaction will proceed
rapidly, but the equilibilum conversion will be close to zero. Consequently, very
little product will be formed. A plot of the equilibrium conversion and the con-
version calculated from the adiabatic energy balance,
is shown in Figure 8- 1 1. We see that for an entering temperature of 600 K the
adiabatic equilibrium conversion is 0.15. The corresponding conversion profile
down the length of the reactor is shown in Figure 8-12. We see that because of
the high entering temperature the rate is very rapid and equilibrium is achieved
very near the reactor entrance.
We notice that the conversion and temperature increase very rapidly over
a short distance (i.e., a small amount of catalyst). This sharp increase is some-
times referred to as the pointltemperature at which the reaction ignites. If the
inlet temperature were lowered to 500 K, the corresponding equilibrium conver-
sion is increased to 0.33; however, the reaction rate is slower at this lower tem-
perature, so that this conversion is not achieved until close to the end of the