Page 507 - Elements of Chemical Reaction Engineering Ebook
P. 507
478 Steady-State Nonisothermal Reactor Design Chap. 8
I 2
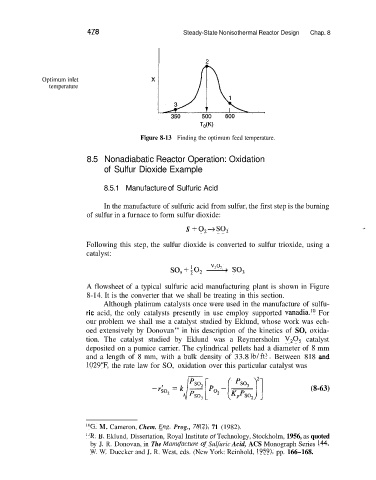
Optimum inlet
temperature
TOW)
Figure 8-13 Finding the optimum feed temperature.
8.5 Nonadiabatic Reactor Operation: Oxidation
of Sulfur Dioxide Example
8.5.1 Manufacture of Sulfuric Acid
In the manufacture of sulfuric acid from sulfur, the first step is the burning
of sulfur in a furnace to form sulfur dioxide:
s +023SO,
Following this step, the sulfur dioxide is converted to sulfur trioxide, using a
catalyst:
so, + 10, ",Os, so3
A flowsheet of a typical sulfuric acid manufacturing plant is shown in Figure
8-14. It is the converter that we shall be treating in this section.
Although platinum catalysts once were used in the manufacture of sulfu-
ric acid, the only catalysts presently in use employ supported vanadia.'* For
our problem we shall use a catalyst studied by Eklund, whose work was ech-
oed extensively by Donovan" in his description of the kinetics of SO, oxida-
tion. The catalyst studied by Eklund was a Reymersholm V,O, catalyst
deposited on a pumice carrier. The cylindrical pellets had a diameter of 8 mm
and a length of 8 mm, with a bulk density of 33.8 lb/ft3. Between 818 and
1029"F, the rate law for SO, oxidation over this particular catalyst was
(8-63)
'OG. M. Cameron, Chem. Eng. Prog., 78(2), 71 (1982).
"R. B. Eklund, Dissertation, Royal Institute of Technology, Stockholm, 1956, as quoted
by J. R. Donovan, in The Manufacture of Sulfuric Acid, ACS Monograph Series 14,
W. W. Duecker and J. R. West, eds. (New York: Reinhold, 1959), pp. 166-168.